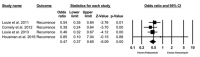Fidaxomicin vs Vancomycin for the Treatment of a First Episode of Clostridium Difficile Infection: A Meta-analysis and Systematic Review
- PMID: 30112254
- PMCID: PMC6089486
- DOI: 10.7759/cureus.2778
Fidaxomicin vs Vancomycin for the Treatment of a First Episode of Clostridium Difficile Infection: A Meta-analysis and Systematic Review
Abstract
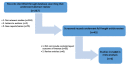
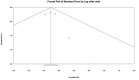
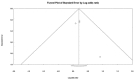
Clostridium difficile infection (CDI) continues to possess a significant disease burden in the United States (US) as well as all over the world. Given the increase in severity and recurrence rate, the decrease in cure rate, and the fact that the virulent ribotype 027 strain remains one of the most commonly identified strains in the US, the Infectious Diseases Society of America (IDSA) published a clinical practice guideline in February 2018 moving away from metronidazole as the first-line treatment for initial CDI and recommending either oral vancomycin or fidaxomicin. The aim of this study is to evaluate the clinical data available comparing the efficacy of primary treatment of CDI between those two antibiotics. We performed a PubMed, PubMed Central, and ScienceDirect database search without restriction to regions, publication types, or languages. A comprehensive literature search was performed from January 1, 1980 up to March 20, 2018. We used the following keywords in different combinations: Clostridium difficile, Clostridium difficile infection, CDI, C. diff, C. difficile, fidaxomicin, vancomycin, pseudomembranous colitis, and antibiotic-associated colitis. The search was limited to human studies. Data were independently extracted by two reviewers with disagreements resolved by a third author. We pooled an odds ratio (OR) on two primary outcomes: Clinical cure rate and rate of recurrence during the follow-up period. The computer search was also supplemented with manual searches by the authors of the retrieved review articles and primary studies. The search phrase "((Clostridium difficile) AND vancomycin) AND fidaxomicin" had the highest yield results. We identified four observational studies with a total of 2,303 patients with CDI that met our inclusion criteria. Compared with vancomycin, fidaxomicin use was associated with a significantly lower recurrence of CDI with a pooled OR of 0.47 (95% confidence interval (CI), 0.37 - 0.60, I2 = 0). On the other hand, there was no significant association of fidaxomicin use with CDI cure rate compared to vancomycin with a pooled OR of 1.22 (95% CI, 0.93 - 1.60, I2 = 0). In light of the recently updated clinical practice guidelines by the IDSA, our review suggests that fidaxomicin has a more sustained clinical response with a statistically significant lower recurrence rate. Although fidaxomicin appears to be the better drug with statistical significance, its cost-effectiveness continues to be an ongoing controversy. More randomized clinical trials are needed to shed light on this matter to assess if there is any clinical significance in fidaxomicin superiority.
Keywords: c. diff; c. difficile; clostridium difficile; fidaxomicin; treatment of clostridium difficile; vancomycin.
Conflict of interest statement
This work has been submitted to the American College of Gastroenterology Conference 2018 in abstract form. This manuscript is not previously published or currently under consideration for publication elsewhere and, if accepted, will not be published elsewhere without written consent of the publisher
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous