Radiological findings of complications after lung transplantation
- PMID: 30112676
- PMCID: PMC6206387
- DOI: 10.1007/s13244-018-0647-9
Radiological findings of complications after lung transplantation
Abstract
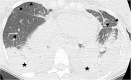
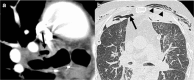
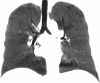
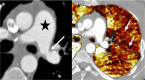
Complications following lung transplantation may impede allograft function and threaten patient survival. The five main complications after lung transplantation are primary graft dysfunction, post-surgical complications, alloimmune responses, infections, and malignancy. Primary graft dysfunction, a transient ischemic/reperfusion injury, appears as a pulmonary edema in almost every patient during the first three days post-surgery. Post-surgical dysfunction could be depicted on computed tomography (CT), such as bronchial anastomosis dehiscence, bronchial stenosis and bronchomalacia, pulmonary artery stenosis, and size mismatch. Alloimmune responses represent acute rejection or chronic lung allograft dysfunction (CLAD). CLAD has three different forms (bronchiolitis obliterans syndrome, restrictive allograft syndrome, acute fibrinoid organizing pneumonia) that could be differentiated on CT. Infections are different depending on their time of occurrence. The first post-operative month is mostly associated with bacterial and fungal pathogens. From the second to sixth months, viral pneumonias and fungal and parasitic opportunistic infections are more frequent. Different patterns according to the type of infection exist on CT. Malignancy should be depicted and corresponded principally to post-transplantation lymphoproliferative disease (PTLD). In this review, we describe specific CT signs of these five main lung transplantation complications and their time of occurrence to improve diagnosis, follow-up, medical management, and to correlate these findings with pathology results. KEY POINTS: • The five main complications are primary graft dysfunction, surgical, alloimmune, infectious, and malignancy complications. • CT identifies anomalies in the setting of unspecific symptoms of lung transplantation complications. • Knowledge of the specific CT signs can allow a prompt diagnosis. • CT signs maximize the yield of bronchoscopy, transbronchial biopsy, and bronchoalveolar lavage. • Radiopathological correlation helps to understand CT signs after lung transplantation complications.
Keywords: Lung transplant complications; Radiological findings.
Conflict of interest statement
The local institutional review board approved the study.
Figures

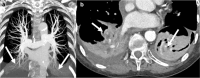
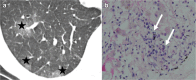
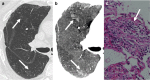
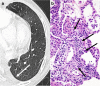
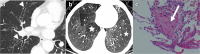

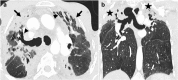
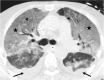
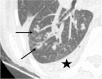
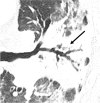
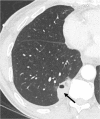
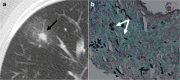
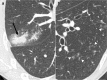
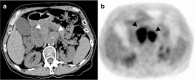
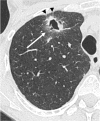
References
-
- Yusen RD, Edwards LB, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1170–1184. doi: 10.1016/j.healun.2016.09.001. - DOI - PubMed
-
- Lund LH, Khush KK, Cherikh WS, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1037–1046. doi: 10.1016/j.healun.2017.07.019. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

