Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials
- PMID: 30113497
- PMCID: PMC6113026
- DOI: 10.1097/MD.0000000000011936
Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials
Abstract
Background: Recently, immune checkpoint inhibitors have shown survival advantage over chemotherapy in the treatment of advanced non-small cell lung cancer (NSCLC). This meta-analysis was conducted to gather and analyze the available evidence (Evidence level I; Randomized Controlled Trials) comparing efficacy and safety of anti-programmed cell death-1 (PD1)/programmed cell death ligand 1 (PD-L1) therapies and chemotherapy in the treatment of advanced NSCLC.
Methods: A search strategy was devised to identify the randomized controlled trials (RCTs) using electronic databases of PubMed, Cochrane Library, and Web of Science. Hazard ratios or odds ratios obtained for overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and treatment related adverse events (TRAEs) were analyzed using fixed effect model or random effects model. Additionally, subgroup analysis was also performed.
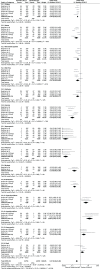
Results: A total of seven RCTs (n = 3867) were identified and selected for inclusion in this meta-analysis. Anti-PD1/PD-L1 therapies (nivolumab, pembrolizumab, atezolizumab) resulted in better OS (HR 0.72 [95% confidence interval [CI] 0.63, 0.82; P < .00001]), PFS (HR 0.84 [95% CI 0.72, 0.97; P < .02]), and ORR (odds ratio [OR] 1.52 [95% CI 1.08, 2.14; P < .02]) in comparison to chemotherapy in advanced NSCLC. Improved safety was observed with anti-PD1/PD-L1 therapies (OR 0.31 [95%CI 0.26, 0.38; P < .00001]). Subgroups analysis revealed Eastern Cooperative Oncology Group Performance Status (ECOG PS) 1 (HR 0.76 [95%CI 0.62, 0.93; P = .007]), squamous cell type (HR 0.76 [95% CI 0.63, 0.92; P = .005]), current/former smoker (HR 0.76 [95% CI 0.63, 0.92; P = .005]), epidermal growth factor receptor (EGFR) wild type (HR 0.67 [95% CI 0.60, 0.76; P < .00001]), Kirsten rat sarcoma oncogene mutation (KRAS) mutant (HR 0.60 [95% CI 0.39, 0.93; P < .02]), and absence of central nervous system (CNS) metastases (HR 0.71 [95% CI 0.63, 0.80; P < .00001]) were associated with better overall survival.
Conclusions: Anti-PD1/PD-L1 therapies are safe and effective treatment option in advanced non-small cell lung cancer and can be recommended selectively.
Conflict of interest statement
The authors declare no conflict of interest in preparing this article.
Figures
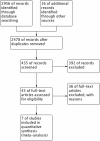


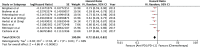


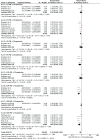
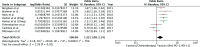

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. - PubMed
-
- American Cancer Society, Cancer facts & Figures 2018. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts... (Accessed Jan 10, 2018).
-
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97. - PubMed
-
- Kubota K, Watanabe K, Kunitoh H, et al. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol 2004;22:254–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

