Calcium/calmodulin-dependent protein kinase IV (CaMKIV) activation contributes to the pathogenesis of experimental colitis via inhibition of intestinal epithelial cell proliferation
- PMID: 30113881
- PMCID: PMC6355073
- DOI: 10.1096/fj.201800535R
Calcium/calmodulin-dependent protein kinase IV (CaMKIV) activation contributes to the pathogenesis of experimental colitis via inhibition of intestinal epithelial cell proliferation
Abstract
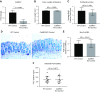
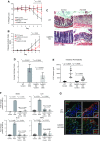
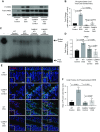
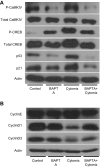
The incidence and prevalence of inflammatory bowel disease (IBD) are increasing worldwide. IBD is known to be multifactorial, but inflammatory signaling within the intestinal epithelium and a subsequent failure of the intestinal epithelial barrier have been shown to play essential roles in disease pathogenesis. CaMKIV is a multifunctional protein kinase associated with inflammation and cell cycle regulation. CaMKIV has been extensively studied in autoimmune diseases, but a role in idiopathic intestinal inflammation has not been described. In this study, active CaMKIV was highly expressed within the intestinal epithelium of humans with ulcerative colitis and wild-type (WT) mice with experimental induced colitis. Clinical disease severity directly correlates with CaMKIV activation, as does expression of proinflammatory cytokines and histologic features of colitis. In WT mice, CaMKIV activation is associated with increases in expression of 2 cell cycle proarrest signals: p53 and p21. Cell cycle arrest inhibits proliferation of the intestinal epithelium and ultimately results in compromised intestinal epithelial barrier integrity, further perpetuating intestinal inflammation during experimental colitis. Using a CaMKIV null mutant mouse, we demonstrate that a loss of CaMKIV protects against murine DSS colitis. Small molecules targeting CaMKIV activation may provide therapeutic benefit for patients with IBD.-Cunningham, K. E., Novak, E. A., Vincent, G., Siow, V. S., Griffith, B. D., Ranganathan, S., Rosengart, M. R., Piganelli, J. D., Mollen, K. P. Calcium/calmodulin-dependent protein kinase IV (CaMKIV) activation contributes to the pathogenesis of experimental colitis via inhibition of intestinal epithelial cell proliferation.
Keywords: CREB; inflammatory bowel disease; intestinal epithelial barrier; intracellular calcium signaling.
Conflict of interest statement
This work was supported, in part, by U.S. National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants K08DK101753 and R03DK114464 (to K.P.M.) and NIH National Institute of General Medical Sciences Grant R01GM082852 (to M.R.R.). The authors declare no conflicts of interest.
Figures



Similar articles
-
Calcium/calmodulin-dependent protein kinase IV signaling pathway is upregulated in experimental necrotizing enterocolitis.Pediatr Surg Int. 2020 Mar;36(3):271-277. doi: 10.1007/s00383-019-04615-w. Epub 2020 Jan 16. Pediatr Surg Int. 2020. PMID: 31950358
-
Repression of Ca2+/calmodulin-dependent protein kinase IV signaling accelerates retinoic acid-induced differentiation of human neuroblastoma cells.J Biol Chem. 2009 Sep 25;284(39):26466-81. doi: 10.1074/jbc.M109.027680. Epub 2009 Jul 24. J Biol Chem. 2009. PMID: 19633294 Free PMC article.
-
Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans.FASEB J. 2016 Aug;30(8):2945-58. doi: 10.1096/fj.201600394R. Epub 2016 Apr 29. FASEB J. 2016. PMID: 27130484 Free PMC article.
-
Calcium/calmodulin-dependent protein kinase IV: A multifunctional enzyme and potential therapeutic target.Prog Biophys Mol Biol. 2016 May;121(1):54-65. doi: 10.1016/j.pbiomolbio.2015.12.016. Epub 2016 Jan 8. Prog Biophys Mol Biol. 2016. PMID: 26773169 Review.
-
CaMK4: Structure, physiological functions, and therapeutic potential.Biochem Pharmacol. 2024 Jun;224:116204. doi: 10.1016/j.bcp.2024.116204. Epub 2024 Apr 12. Biochem Pharmacol. 2024. PMID: 38615920 Review.
Cited by
-
Antioxidants, minerals and vitamins in relation to Crohn's disease and ulcerative colitis: A Mendelian randomization study.Aliment Pharmacol Ther. 2023 Feb;57(4):399-408. doi: 10.1111/apt.17392. Epub 2023 Jan 16. Aliment Pharmacol Ther. 2023. PMID: 36645152 Free PMC article.
-
Ellagic Acid Triggers the Necrosis of Differentiated Human Enterocytes Exposed to 3-Nitro-Tyrosine: An MS-Based Proteomic Study.Antioxidants (Basel). 2022 Dec 17;11(12):2485. doi: 10.3390/antiox11122485. Antioxidants (Basel). 2022. PMID: 36552693 Free PMC article.
-
Nix-Mediated Mitophagy Modulates Mitochondrial Damage During Intestinal Inflammation.Antioxid Redox Signal. 2020 Jul 1;33(1):1-19. doi: 10.1089/ars.2018.7702. Epub 2020 Mar 31. Antioxid Redox Signal. 2020. PMID: 32103677 Free PMC article.
-
Mechanism of Acupuncture and Moxibustion on Promoting Mucosal Healing in Ulcerative Colitis.Chin J Integr Med. 2023 Sep;29(9):847-856. doi: 10.1007/s11655-022-3531-x. Epub 2022 Apr 12. Chin J Integr Med. 2023. PMID: 35412218 Review.
-
Anterior Gradient Protein 2 Promotes Mucosal Repair in Pediatric Ulcerative Colitis.Biomed Res Int. 2021 May 18;2021:6483860. doi: 10.1155/2021/6483860. eCollection 2021. Biomed Res Int. 2021. PMID: 34055987 Free PMC article.
References
-
- Greco C. A., Maurer-Spurej E., Scott M. D., Kalab M., Nakane N., Ramírez-Arcos S. M. (2011) PEGylation prevents bacteria-induced platelet activation and biofilm formation in platelet concentrates. Vox Sang. 100, 336–339 - PubMed
-
- Crohn’s & Colitis Foundation of America. (2014) The Facts about Inflammatory Bowel Disease, Crohn’s & Colitis Foundation of America, New York. Accessed January, 18, 2018, at http://www.crohnscolitisfoundation.org/assets/pdfs/updatedibdfactbook.pdf/
-
- Frolkis A. D., Dykeman J., Negrón M. E., Debruyn J., Jette N., Fiest K. M., Frolkis T., Barkema H. W., Rioux K. P., Panaccione R., Ghosh S., Wiebe S., Kaplan G. G. (2013) Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 145, 996–1006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

