Using stratified medicine to understand, diagnose, and treat neuropathic pain
- PMID: 30113945
- PMCID: PMC6130809
- DOI: 10.1097/j.pain.0000000000001301
Using stratified medicine to understand, diagnose, and treat neuropathic pain
Conflict of interest statement
DLB has undertaken consultancy and advisory board work for Oxford innovation—in the past 36 months, this has included work for Abide, Biogen, GSK, Lilly, Mitsubishi Tanabe, Mundipharma, Teva, and Pfizer.
Figures
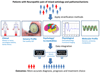
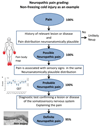


References
-
- Attal N, Bouhassira D, Baron R. Diagnosis and assessment of neuropathic pain through questionnaires. The Lancet Neurology. 2018;17(5):456–466. - PubMed
-
- Attal N, de Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, Raicher I, Uceyler N, Sommer C, Bouhassira D. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. The Lancet Neurology. 2016;15(6):555–565. - PubMed
-
- Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. The Lancet Neurology. 2012;11(11):999–1005. - PubMed
-
- Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. PAIN. 2009;146(1–2):34–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

