Tolerance of preterm formula versus pasteurized donor human milk in very preterm infants: a randomized non-inferiority trial
- PMID: 30115086
- PMCID: PMC6097280
- DOI: 10.1186/s13052-018-0532-7
Tolerance of preterm formula versus pasteurized donor human milk in very preterm infants: a randomized non-inferiority trial
Abstract
Background: Human milk (HM) is the best feeding for premature infants. When own mother's milk (OMM) is insufficient or unavailable, pasteurized donor human milk (PDHM) and preterm formula (PF) are the alternative nutritional sources, but the benefits of donor milk over formula are not defined. This study aimed to assess whether, in the absence of OMM, the PF could guarantee a feeding tolerance not inferior to that seen with the use of PDHM during the first two weeks of life of very preterm infants.
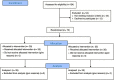
Methods: Infants with gestational age (GA) of ≤32 weeks who started enteral feeding within the first 7 days of life were randomized to receive PDHM or PF as a supplement to the OMM insufficient or unavailable. The primary outcome was the day of life when full enteral feeding (FEF) of 150 mL/Kg/d was achieved.
Results: Seventy infants were randomized, 35 in the PF group (GA 30.2 ± 1.7 weeks; BW 1342 ± 275 g), 35 in the PDHM group (GA 30 ± 1.9 weeks; BW 1365 ± 332 g). The time to achieve FEF was the same for infants fed with PF and for infants fed with PDHM (12.3 ± 7.0 days vs 12.8 ± 6.5).
Conclusions: This trial shows that PF could be a valid alternative for the early feeding of very preterm infants when OMM is insufficient or unavailable.
Trial registration: UMIN000013922 . Date of formal registration: December 31, 2014.
Keywords: Feeding tolerance; Pasteurized donor human milk; Preterm formula; Very preterm infant.
Conflict of interest statement
Ethics approval and consent to participate
The institutional review boards approved the study, and written informed consent was obtained from the parents of all subjects before enrollment.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Hallowell SG, Rogowski JA, Spatz DL, Hanlon AL, Kenny M, Lake ET. Factors associated with infant feeding of human milk at discharge from neonatal intensive care: cross-sectional analysis of nurse survey and infant outcomes data. Int J Nurs Stud. 2016;53:190. doi: 10.1016/j.ijnurstu.2015.09.016. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical