Metacognitive therapy home-based self-help for cardiac rehabilitation patients experiencing anxiety and depressive symptoms: study protocol for a feasibility randomised controlled trial (PATHWAY Home-MCT)
- PMID: 30115112
- PMCID: PMC6097432
- DOI: 10.1186/s13063-018-2826-x
Metacognitive therapy home-based self-help for cardiac rehabilitation patients experiencing anxiety and depressive symptoms: study protocol for a feasibility randomised controlled trial (PATHWAY Home-MCT)
Abstract
Background: Anxiety and depression are common among patients attending cardiac rehabilitation services. Currently available pharmacological and psychological interventions have limited effectiveness in this population. There are presently no psychological interventions for anxiety and depression integrated into cardiac rehabilitation services despite emphasis in key UK National Health Service policy. A new treatment, metacognitive therapy, is highly effective at reducing anxiety and depression in mental health settings. The principal aims of the current study are (1) to evaluate the acceptability of delivering metacognitive therapy in a home-based self-help format (Home-MCT) to cardiac rehabilitation patients experiencing anxiety and depressive symptoms and conduct a feasibility trial of Home-MCT plus usual cardiac rehabilitation compared to usual cardiac rehabilitation; and (2) to inform the design and sample size for a full-scale trial.
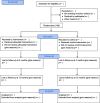
Methods: The PATHWAY Home-MCT trial is a single-blind feasibility randomised controlled trial comparing usual cardiac rehabilitation (control) versus usual cardiac rehabilitation plus home-based self-help metacognitive therapy (intervention). Economic and qualitative evaluations will be embedded within the trial. Participants will be assessed at baseline and followed-up at 4 and 12 months. Patients who have been referred to cardiac rehabilitation programmes and have a score of ≥ 8 on the anxiety and/or depression subscales of the Hospital Anxiety and Depression Scale will be invited to take part in the study and written informed consent will be obtained. Participants will be recruited from the National Health Service in the UK. A minimum of 108 participants will be randomised to the intervention and control arms in a 1:1 ratio.
Discussion: The Home-MCT feasibility randomised controlled trial will provide evidence on the acceptability of delivering metacognitive therapy in a home-based self-help format for cardiac rehabilitation patients experiencing symptoms of anxiety and/or depression and on the feasibility and design of a full-scale trial. In addition, it will provide provisional point estimates, with appropriately wide measures of uncertainty, relating to the effectiveness and cost-effectiveness of the intervention.
Trial registration: ClinicalTrials.gov, NCT03129282 , Submitted to Registry: 11 April 2017.
Keywords: Anxiety; Cardiac rehabilitation; Depression; Heart disease; Home therapy; Metacognitive therapy; Psychological intervention; Rumination; Self-help; Worry.
Conflict of interest statement
Ethics approval and consent to participate
This trial received full ethical approval from the North West - Greater Manchester West Research Ethics Committee on November 17, 2016, Research Ethics Committee (REC) Reference 16/NW/0786, IRAS ID 186990. The following two participating NHS sites have approved the study: Aintree University Hospitals NHS Foundation Trust and Bolton NHS Foundation Trust.
The trial has been registered in
Any important modifications to the protocol will be submitted for further ethical approval and approved changes will be documented and communicated to the REC, trial registry, steering committee and all relevant parties. The study will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki (1996), the principles of Good Clinical Practice, and the UK Policy Framework for Health and Social Care Research (2017).
GMMH acts as the sponsor for this study. As the sponsor is an NHS organisation, the NHS indemnity scheme will apply
Written informed consent will be obtained from all participants in the study. Participants will be asked to complete three original copies of the consent form, one will be given to them, the other one will be kept in the site file and the other will be filed on the patients’ medical notes. Participants will be free to withdraw from the study at any time without giving a reason and without their care being affected. All the information collected during this trial will be confidential and held in accordance with NHS Data Protection guidelines and Good Clinical Practice guidelines. Confidentiality will only be breached if participants disclose information which may indicate that there is a risk of harm to themselves or others. Every opportunity to discuss any possible breaches of confidentiality with participants will be taken prior to informing any appropriate agencies, e.g. cardiac service staff, GP or A&E services.
All researchers and study site staff involved with the study must comply with the requirements of the Data Protection Act 1998 regarding the collection, storage, processing and disclosure of personal information, and will uphold the Act’s core principles.
Audio-recordings and transcriptions of interviews will be stored on NHS drives, which are password protected and designed for the storage of confidential research material.
Interviews which are transcribed will be anonymised at the point of transcription. Any third party involved with transcribing of interviews will sign a confidentiality agreement and be fully instructed in how to anonymise transcripts.
Documents will be maintained by the sponsor (GMMH) and at the Investigator Sites in a way that will facilitate the management of the study, audit and inspection. Investigator site files will return to the sponsor after recruitment ends. Storage of data within GMMH will follow the standard operating procedure, which states that all information involving NHS patients, interventional or observational, should be stored for a minimum of 15 years after completion of the study in a secure storage area with limited access. GMMH will arrange appropriate storage and archiving of data within the research and innovation department. Access to study documents will be restricted to authorised persons.
The study end date is deemed to be the date of the last data capture. The Chief Investigator has the right at any time to terminate the study for clinical or administrative reasons.
The end of the study will be reported to the Research Ethics Committee (REC) within the required timeframe if the study is terminated prematurely. Investigators will inform participants and sites of any premature termination of the study and ensure that the appropriate follow-up is arranged for all involved. Following the end of the study, a summary report of the study will be provided to the REC within the required timeframe.
Consent for publication
Not applicable.
Competing interests
Professor Adrian Wells is the developer of MCT and co-director of the MCT Institute:
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Naylor C, Parsonage M, McDaid D, Knapp M, Fossey M, Galea A. Long-term Conditions and Mental Health - The Cost of Co-morbidities. King's Fund Centre Mental Health 2012. https://www.kingsfund.org.uk/publications/long-term-conditions-and-menta.... Accessed 6 Nov 2017.
-
- Tully PJ, Cosh SM, Baumeister H. The anxious heart in whose mind? A systematic review and meta-regression of factors associated with anxiety disorder diagnosis, treatment and morbidity risk in coronary heart disease. J Psychosom Res. 2014;77(6):439–448. doi: 10.1016/j.jpsychores.2014.10.001. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

