Harmonization of Liquid Chromatography-Tandem Mass Spectrometry Protein Assays
- PMID: 30115394
- PMCID: PMC6921243
- DOI: 10.1016/j.cll.2018.05.004
Harmonization of Liquid Chromatography-Tandem Mass Spectrometry Protein Assays
Abstract
Harmonization of diagnostic test results is fundamental to the effective use of laboratory testing in the diagnosis, treatment, and monitoring of disease. Formal approaches to harmonization and standardization provide a rigorous and high-quality roadmap to this end, although the formal harmonization process can be long and complex. In the meantime, more informal approaches to harmonization can provide a useful pathway to improved harmonization in the short term. Factors relevant to harmonization are discussed with particular attention to protein assays using LC-MS/MS. Published formal and informal harmonization projects are provided as examples, including lessons drawn from these projects.
Keywords: Harmonization; LC-MS/MS; Protein; Standardization.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures: A.L. Rockwood and M.S. Lowenthal have no conflicts to disclose. C. Bystrom is an employee of Cleveland HeartLab.
Figures
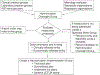



References
-
- AACC POSITION STATEMENT - Harmonization of clinical laboratory test results [Web site], 2013. Available at: https://www.harmonization.net/media/1087/aacc_harmonization_position_sta.... Accessed June 7, 2018.
-
- Greg Miller W, Myers GL, Lou Gantzer M, et al. Roadmap for harmonization of clinical laboratory measurement procedures. Clin Chem 2011;57(8):1108–17. - PubMed
-
- Phinney KW, Sempos CT, Tai SS, et al. Baseline assessment of 25-Hydroxyvitamin D reference material and proficiency testing/external quality assurance material commutability: a vitamin D standardization program study. J AOAC Int 2017; 100(5):1288–93. - PubMed
-
- Saenger AK, Rodriguez-Fraga O, Ler R, et al. Specificity of B-type natriuretic peptide assays: cross-reactivity with different BNP, NT-proBNP, and proBNP peptides. Clin Chem 2017;63(1):351–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

