Phenotypic plasticity in Periplaneta americana photoreceptors
- PMID: 30115661
- PMCID: PMC6168239
- DOI: 10.1085/jgp.201812107
Phenotypic plasticity in Periplaneta americana photoreceptors
Abstract
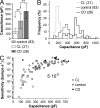
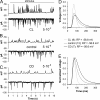
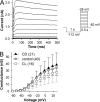
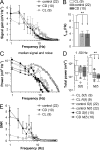
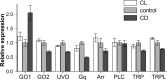
Plasticity is a crucial aspect of neuronal physiology essential for proper development and continuous functional optimization of neurons and neural circuits. Despite extensive studies of different visual systems, little is known about plasticity in mature microvillar photoreceptors. Here we investigate changes in electrophysiological properties and gene expression in photoreceptors of the adult cockroach, Periplaneta americana, after exposure to constant light (CL) or constant dark (CD) for several months. After CL, we observed a decrease in mean whole-cell capacitance, a proxy for cell membrane area, from 362 ± 160 to 157 ± 58 pF, and a decrease in absolute sensitivity. However, after CD, we observed an increase in capacitance to 561 ± 155 pF and an increase in absolute sensitivity. Small changes in the expression of light-sensitive channels and signaling molecules were detected in CD retinas, together with a substantial increase in the expression of the primary green-sensitive opsin (GO1). Accordingly, light-induced currents became larger in CD photoreceptors. Even though normal levels of GO1 expression were retained in CL photoreceptors, light-induced currents became much smaller, suggesting that factors other than opsin are involved. Latency of phototransduction also decreased significantly in CL photoreceptors. Sustained voltage-activated K+ conductance was not significantly different between the experimental groups. The reduced capacitance of CL photoreceptors expanded their bandwidth, increasing the light-driven voltage signal at high frequencies. However, voltage noise was also amplified, probably because of unaltered expression of TRPL channels. Consequently, information transfer rates were lower in CL than in control or CD photoreceptors. These changes in whole-cell capacitance and electrophysiological parameters suggest that structural modifications can occur in the photoreceptors to adapt their function to altered environmental conditions. The opposing patterns of modifications in CL and CD photoreceptors differ profoundly from previous findings in Drosophila melanogaster photoreceptors.
© 2018 Frolov et al.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

