Intrathecal Injection of Dual Zipper Kinase shRNA Alleviating the Neuropathic Pain in a Chronic Constrictive Nerve Injury Model
- PMID: 30115872
- PMCID: PMC6121272
- DOI: 10.3390/ijms19082421
Intrathecal Injection of Dual Zipper Kinase shRNA Alleviating the Neuropathic Pain in a Chronic Constrictive Nerve Injury Model
Abstract
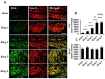
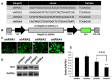
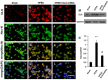
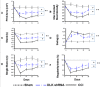
Dual leucine zipper kinase (DLK) is a member of mitogen-activated protein kinase kinase kinase (MAP3K) family mainly involved in neuronal degeneration. However, the role of DLK signaling in the neuropathic pain has not yet been fully determined. Chronic constrictive injury (CCI) was conducted by four 3-0 chromic gut ligatures loosely ligated around the sciatic nerve. Escalated DLK expression over the dorsal root ganglion was observed from one to four rings of CCI. Remarkable expression of DLK was observed in primary dorsal root ganglion cells culture subjected to electrical stimulation and attenuated by DLK short hairpin RNA (shRNA) treatment. Intrathecal injection of DLK shRNA attenuates the expression of DLK over the dorsal root ganglion and hippocampus neurons and increased the threshold of mechanical allodynia and decreased thermal hyperalgesia. In CatWalk gait analysis, significant decreases of print area, maximum contact maximum intensity, stand phase, single stance, and regular index by CCI were alleviated by the DLK shRNA administration. In conclusion, the expression of DLK was up-regulated in chronic constrictive injury and attenuated by the administration of DLK shRNA, which paralleled the improvement of neurobehavior of neuropathic pain. The modulation of DLK expression is a potential clinic treatment option for neuropathic pain.
Keywords: chronic constrictive injury; dual zipper kinase; neuropathic pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Feasibility of Human Amniotic Fluid Derived Stem Cells in Alleviation of Neuropathic Pain in Chronic Constrictive Injury Nerve Model.PLoS One. 2016 Jul 21;11(7):e0159482. doi: 10.1371/journal.pone.0159482. eCollection 2016. PLoS One. 2016. PMID: 27441756 Free PMC article.
-
Comprehensive analysis of neurobehavior associated with histomorphological alterations in a chronic constrictive nerve injury model through use of the CatWalk XT system.J Neurosurg. 2014 Jan;120(1):250-62. doi: 10.3171/2013.9.JNS13353. Epub 2013 Nov 1. J Neurosurg. 2014. PMID: 24180567
-
Chemokine CXCL10/CXCR3 signaling contributes to neuropathic pain in spinal cord and dorsal root ganglia after chronic constriction injury in rats.Neurosci Lett. 2019 Feb 16;694:20-28. doi: 10.1016/j.neulet.2018.11.021. Epub 2018 Nov 15. Neurosci Lett. 2019. PMID: 30448292
-
Regulation of the Activity of the Dual Leucine Zipper Kinase by Distinct Mechanisms.Cells. 2024 Feb 11;13(4):333. doi: 10.3390/cells13040333. Cells. 2024. PMID: 38391946 Free PMC article. Review.
-
Deciphering the multifunctional role of dual leucine zipper kinase (DLK) and its therapeutic potential in disease.Eur J Med Chem. 2023 Jul 5;255:115404. doi: 10.1016/j.ejmech.2023.115404. Epub 2023 Apr 20. Eur J Med Chem. 2023. PMID: 37098296 Review.
Cited by
-
Modulation of Aryl Hydrocarbon Receptor Expression Alleviated Neuropathic Pain in a Chronic Constriction Nerve Injury Animal Model.Int J Mol Sci. 2022 Sep 24;23(19):11255. doi: 10.3390/ijms231911255. Int J Mol Sci. 2022. PMID: 36232555 Free PMC article.
-
Multitasking: Dual Leucine Zipper-Bearing Kinases in Neuronal Development and Stress Management.Annu Rev Cell Dev Biol. 2019 Oct 6;35:501-521. doi: 10.1146/annurev-cellbio-100617-062644. Annu Rev Cell Dev Biol. 2019. PMID: 31590586 Free PMC article. Review.
References
-
- Treede R.D., Jensen T.S., Campbell J.N., Cruccu G., Dostrovsky J.O., Griffin J.W., Hansson P., Hughes R., Nurmikko T., Serra J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. - DOI - PubMed
-
- Jin S.X., Zhuang Z.Y., Woolf C.J., Ji R.R. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

