Endotoxins from a Pharmacopoeial Point of View
- PMID: 30115887
- PMCID: PMC6115822
- DOI: 10.3390/toxins10080331
Endotoxins from a Pharmacopoeial Point of View
Abstract
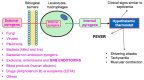
A pyrogen is a substance that causes fever after intravenous administration or inhalation. Gram negative endotoxins are the most important pyrogens to pharmaceutical laboratories. In the International, United States, Japanese and European Pharmacopoeias, there are two official methods to evaluate pyrogenicitythat is, the bacterial endotoxin test, and the pyrogen test. The main objective of this review is to compare the monographs of each test among the different Pharmacopeias, to detect similarities and differences. The former can be considered fully harmonized, and only non-significant differences were detected. The latter, which is the only available assay for some products and formulations to demonstrate apyrogenicity, shows large differences, which should be considered.
Keywords: endotoxins; harmonization; parenteral drug products; pharmacopoeial test; pyrogens.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Detection of endotoxins and other pyrogens using human whole blood.Dev Biol Stand. 1999;101:131-9. Dev Biol Stand. 1999. PMID: 10566786
-
Relative potency of "environmental" endotoxin as measured by the Limulus amebocyte lysate test and the USP rabbit pyrogen test.Prog Clin Biol Res. 1982;93:65-77. Prog Clin Biol Res. 1982. PMID: 7122606 No abstract available.
-
Comparison of commercial pyrogen testing laboratories.Am J Hosp Pharm. 1976 Aug;33(8):801-3. Am J Hosp Pharm. 1976. PMID: 949064
-
[Application of a bacterial endotoxin test for parenteral drugs].Eisei Shikenjo Hokoku. 1994;(112):209-11. Eisei Shikenjo Hokoku. 1994. PMID: 8854936 Review. Japanese.
-
Standardized pyrogen testing of medical products with the bacterial endotoxin test (BET) as a substitute for rabbit Pyrogen testing (RPT): A scoping review.Toxicol In Vitro. 2021 Aug;74:105160. doi: 10.1016/j.tiv.2021.105160. Epub 2021 Apr 6. Toxicol In Vitro. 2021. PMID: 33831473
Cited by
-
Heparin enables the reliable detection of endotoxin in human serum samples using the Limulus amebocyte lysate assay.Sci Rep. 2024 Jan 29;14(1):2410. doi: 10.1038/s41598-024-52735-8. Sci Rep. 2024. PMID: 38287051 Free PMC article.
-
Physicochemical characterization of biological and synthetic forms of two lipid A-based TLR4 agonists.Heliyon. 2023 Jul 8;9(7):e18119. doi: 10.1016/j.heliyon.2023.e18119. eCollection 2023 Jul. Heliyon. 2023. PMID: 37483830 Free PMC article.
-
Microbiological Contamination of Medicinal Products -Is It a Significant Problem?Pharmaceuticals (Basel). 2025 Jun 23;18(7):946. doi: 10.3390/ph18070946. Pharmaceuticals (Basel). 2025. PMID: 40732236 Free PMC article. Review.
-
Aptamer-based biosensors for the diagnosis of sepsis.J Nanobiotechnology. 2021 Jul 19;19(1):216. doi: 10.1186/s12951-021-00959-5. J Nanobiotechnology. 2021. PMID: 34281552 Free PMC article. Review.
-
A Study on the Application of Recombinant Factor C (rFC) Assay Using Biopharmaceuticals.Microorganisms. 2024 Mar 4;12(3):516. doi: 10.3390/microorganisms12030516. Microorganisms. 2024. PMID: 38543567 Free PMC article.
References
-
- Sandel T. Assesing non-endotoxin microbial pyrogens in relation in pharmaceutical processing. J. GXP Compliance. 2015;19:1–12.
-
- Tours N., Sandle T. Comparison of dry-heat depyrogenation using three different types of Gram-negative bacterial endotoxin. Eur. J. Parenter. Pharm. Sci. 2008;13:17–20.
-
- Ensor D.S., Foarde K.K. The behavior of particles in cleanrooms. In: Dixon A.M., editor. Environmental Monitoring for Cleanrooms and Controlled Environments. 1st ed. CRC Press Taylor & Francis; Boca Raton, FL, USA: 2016. pp. 1–28.
-
- Seibert F.B. The cause of many febrile reactions following intravenous injections. I. Am. J. Physiol. 1925;71:621–651. doi: 10.1152/ajplegacy.1925.71.3.621. - DOI
-
- Welch H., Calvery H.D., McClosky W.T., Price C.W. Method of preparation and test for bacterial pyrogens. J. Am. Pharm. Assoc. 1943;32:65–69. doi: 10.1002/jps.3030320301. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases