Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma
- PMID: 30115903
- PMCID: PMC6095846
- DOI: 10.1038/s12276-018-0136-8
Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma
Abstract
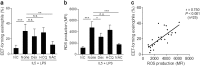
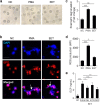
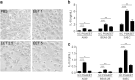
Eosinophil extracellular traps (EETs), a complex of DNA fibers and cytotoxic granule proteins, are implicated in the development of asthma; however, the pathophysiological function of EETs in immune responses has not been fully determined. The present study investigated the characteristics of EETs from patients with non-severe asthma (NSA, n = 20) and severe eosinophilic asthma (SEA, n = 20) and evaluated EET function. The percentage of EET-forming peripheral blood eosinophils stimulated with IL-5 and LPS was significantly higher in patients with SEA than in those with NSA (P = 0.009). This percentage negatively correlated with baseline FEV1 (r = -0.350, P = 0.027) and positively correlated with serum eosinophil-derived neurotoxin levels in asthmatic subjects (r = 0.437, P = 0.018). In addition, EET formation was markedly associated with reactive oxygen species production (r = 0.750, P < 0.001). These EETs exhibited an autocrine function to induce eosinophil degranulation, which led to granule protein production. Airway epithelial cells stimulated with EETs exhibited increased epithelial detachment and permeability and pro-inflammatory cytokine release. However, EETs were not significantly associated with mast cell activation. The present study suggests that peripheral blood eosinophils from patients with SEA may be more activated to produce EETs than those from patients with NSA, which further induces inflammation in asthmatic airways. Therefore, regulation of EET formation and function may be a novel therapeutic approach for asthma management.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma.Clin Exp Allergy. 2017 Jan;47(1):57-70. doi: 10.1111/cea.12859. Clin Exp Allergy. 2017. PMID: 27883241
-
Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma.Allergy. 2020 Jan;75(1):95-103. doi: 10.1111/all.13997. Epub 2019 Nov 8. Allergy. 2020. PMID: 31330043
-
Lysophosphatidylserine induces eosinophil extracellular trap formation and degranulation: Implications in severe asthma.Allergy. 2020 Dec;75(12):3159-3170. doi: 10.1111/all.14450. Epub 2020 Jul 10. Allergy. 2020. PMID: 32535937
-
Eosinophil extracellular traps in asthma: implications for pathogenesis and therapy.Respir Res. 2023 Sep 26;24(1):231. doi: 10.1186/s12931-023-02504-4. Respir Res. 2023. PMID: 37752512 Free PMC article. Review.
-
IL-5 and IL-5 receptor in asthma.Mem Inst Oswaldo Cruz. 1997;92 Suppl 2:75-91. doi: 10.1590/s0074-02761997000800012. Mem Inst Oswaldo Cruz. 1997. PMID: 9698919 Review.
Cited by
-
Combined IgE neutralization and Bifidobacterium longum supplementation reduces the allergic response in models of food allergy.Nat Commun. 2022 Sep 27;13(1):5669. doi: 10.1038/s41467-022-33176-1. Nat Commun. 2022. PMID: 36167830 Free PMC article.
-
Citrullinated histone H3, a marker of extracellular trap formation, is increased in blood of stable asthma patients.Clin Transl Allergy. 2020 Jul 13;10:31. doi: 10.1186/s13601-020-00337-8. eCollection 2020. Clin Transl Allergy. 2020. PMID: 32685129 Free PMC article.
-
Role of Eosinophils in Intestinal Inflammation and Fibrosis in Inflammatory Bowel Disease: An Overlooked Villain?Front Immunol. 2021 Oct 19;12:754413. doi: 10.3389/fimmu.2021.754413. eCollection 2021. Front Immunol. 2021. PMID: 34737752 Free PMC article. Review.
-
Which Factors Associated With Activated Eosinophils Contribute to the Pathogenesis of Aspirin-Exacerbated Respiratory Disease?Allergy Asthma Immunol Res. 2019 May;11(3):320-329. doi: 10.4168/aair.2019.11.3.320. Allergy Asthma Immunol Res. 2019. PMID: 30912322 Free PMC article. Review.
-
Serum Levels of Eosinophil-Derived Neurotoxin: A Biomarker for Asthma Severity in Adult Asthmatics.Allergy Asthma Immunol Res. 2019 May;11(3):394-405. doi: 10.4168/aair.2019.11.3.394. Allergy Asthma Immunol Res. 2019. PMID: 30912328 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

