Association of anxiety with subcortical amyloidosis in cognitively normal older adults
- PMID: 30116029
- PMCID: PMC6377864
- DOI: 10.1038/s41380-018-0214-2
Association of anxiety with subcortical amyloidosis in cognitively normal older adults
Erratum in
-
Correction: Association of anxiety with subcortical amyloidosis in cognitively normal older adults.Mol Psychiatry. 2020 Oct;25(10):2644. doi: 10.1038/s41380-018-0323-y. Mol Psychiatry. 2020. PMID: 30538309 Free PMC article.
Abstract
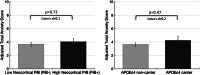
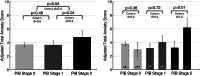
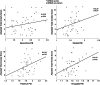
Late-life anxiety has been associated with increased progression from normal cognition to amnestic MCI, suggesting that anxiety may be a neuropsychiatric symptom of Alzheimer's disease (AD) pathological changes and a possible marker of anatomical progression in preclinical AD. This study examined whether cortical or subcortical amyloidosis, indicating earlier or later stages of preclinical AD, was associated with greater self-reported anxiety among 118 cognitively normal volunteers, aged 65-90 years, and whether this association was stronger in APOEε4 carriers. Participants underwent Pittsburgh Compound B Positron Emission Tomography (PiB-PET) to assess fibrillar amyloid-β burden in cortical and subcortical regions, and measurement of anxiety using the Hospital Anxiety and Depression Scale-anxiety subscale. Higher PiB-PET measures in the subcortex (striatum, amygdala, and thalamus), but not in the cortex, were associated with greater anxiety, adjusting for demographics, cognition, and depression. Findings were similar using a cortico-striatal staging system and continuous PET measurements. Anxiety was highest in APOEε4 carriers with subcortical amyloidosis. This work supports in vivo staging of amyloid-β deposition in both cortical and subcortical regions as a promising approach to the study of neuropsychiatric symptoms such as anxiety in cognitively normal older individuals. Elevated anxiety symptoms in combination with high-risk biological factors such as APOEε4 and subcortical amyloid-β may identify participants closest to MCI for secondary prevention trials.
Conflict of interest statement
BJH has served as a paid consultant for GE Healthcare. VJ, JJ, and RAB have no disclosures. DMR has served as a paid consultant for Eli Lilly, Janssen, and Biogen, and sits on the Scientific Advisory Board for Neurotrack. KAJ has served as paid consultant for Biogen, Janssen, Piramal, Novartis, Genetech, Roche, Lundbeck, Eli Lilly, Genzyme, AZtherapies, Abbvie, and Merck. RAS has served as a paid consultant for Abbvie, Biogen, Bracket, Genentech, Lundbeck, Roche, and Sanofi. She has served as a co-investigator for Avid, Eli Lilly, and Janssen. She has spoken at symposia sponsored by Eli Lilly, Biogen, and Janssen. NJD has received salary support from Eisai and Eli Lilly. She has served as a paid consultant to Avanir Pharmaceuticals. Her spouse is employed by Alkermes. None of these relationships are related to the content of the manuscript.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

