Quantitative Recovery of Viable Lactobacillus paracasei CNCM I-1572 (L. casei DG®) After Gastrointestinal Passage in Healthy Adults
- PMID: 30116228
- PMCID: PMC6083036
- DOI: 10.3389/fmicb.2018.01720
Quantitative Recovery of Viable Lactobacillus paracasei CNCM I-1572 (L. casei DG®) After Gastrointestinal Passage in Healthy Adults
Abstract
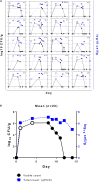
Probiotics are live microorganisms, and viability after transit through the gastrointestinal tract (GIT) is considered an inherent property of the health benefits of probiotics. The aim of the present study was to quantify the viable and total loads of Lactobacillus paracasei DG cells after passage through the GIT following the consumption of the probiotic product Enterolactis (L. casei DG®; L. paracasei CNCM I-1572; L. paracasei DG) from drinkable vials by healthy adults. We developed a novel method for discriminating and enumerating culturable L. paracasei DG cells based on the unique sticky, filamentous phenotype of this strain on MRS agar containing vancomycin and kanamycin. The identity of DG was also confirmed with strain-specific primers by colony PCR. This method was used for a recovery study of the DG strain to quantify viable cells in the fecal samples of 20 volunteers during a 1-week probiotic consumption period and a 1-week follow-up. We isolated L. paracasei DG from at least one fecal sample from all the volunteers. The highest concentration of viable DG cells [ranging from 3.6 to 6.7 log10 colony-forming unit (CFU) per gram of feces] in the feces was observed between 4 and 8 days from the beginning of Enterolactis intake and for up to 5 days after cessation of intake. As expected, the total DG count determined by real-time quantitative PCR (qPCR) was mostly higher than the viable DG cells recovered. Viable count experiments, carried out by combining ad hoc culture-based discriminative conditions and strain-specific molecular biological protocols, unambiguously demonstrated that L. paracasei DG can survive gastrointestinal transit in healthy adults when ingested as Enterolactis in drinkable vials containing no less than one billion CFU at the end of shelf life.
Keywords: EPS; Enterolactis; isolation; probiotic; qPCR.
Figures

References
-
- Balzaretti S., Taverniti V., Guglielmetti S., Fiore W., Minuzzo M., Ngo H. N., et al. (2017). A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl. Environ. Microbiol. 83:e02702-16. 10.1128/AEM.02702-16 - DOI - PMC - PubMed
-
- Compare D., Rocco A., Coccoli P., Angrisani D., Sgamato C., Iovine B., et al. (2017). Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: an ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 17:53. 10.1186/s12876-017-0605-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

