Isolation and Characterization of NP-POL Nonapeptide for Possible Therapeutic Use in Parkinson's Disease
- PMID: 30116478
- PMCID: PMC6079319
- DOI: 10.1155/2018/3760124
Isolation and Characterization of NP-POL Nonapeptide for Possible Therapeutic Use in Parkinson's Disease
Abstract
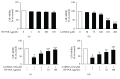
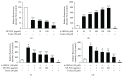
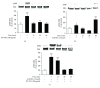
Colostrum and milk are the initial mammalian nourishment and rich reservoir of essential nutrients for newborn development. Bioactive peptides isolated from natural sources, such as colostrum, serve as endogenous regulators and can be used as alternative therapeutic agents in the treatment of neurodegenerative diseases. One example is the previously unknown NP-POL nonapeptide isolated from Colostrinin. In the present study, we investigated a method of NP-POL nonapeptide isolation using Bio-Gel P2 molecular sieve chromatography. We showed the protective effect of NP-POL on 6-hydroxydopamine- (6-OHDA-) induced neurotoxicity using rat adrenal pheochromocytoma (PC12 Tet On) cells. Treatment of PC12 cells with NP-POL nonapeptide reduced 6-OHDA-induced apoptosis and caused transient phosphorylation of extracellular signal-regulated kinases (ERK 1/2), which were shown to promote cell survival. NP-POL nonapeptide also protected neuronal cells against oxidative injury induced by 6-OHDA. These results showed a potential use of NP-POL in the therapy of Parkinson's disease.
Figures



Similar articles
-
Effects of (-)-sesamin on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and dopaminergic neuronal cells of Parkinson's disease rat models.Neurochem Int. 2015 Apr-May;83-84:19-27. doi: 10.1016/j.neuint.2015.01.003. Epub 2015 Mar 3. Neurochem Int. 2015. PMID: 25747493
-
Neuroprotective effects of dimerumic acid and deferricoprogen from Monascus purpureus NTU 568-fermented rice against 6-hydroxydopamine-induced oxidative stress and apoptosis in differentiated pheochromocytoma PC-12 cells.Pharm Biol. 2016 Aug;54(8):1434-44. doi: 10.3109/13880209.2015.1104698. Epub 2016 Jan 21. Pharm Biol. 2016. PMID: 26794209
-
Effects of the hook of Uncaria rhynchophylla on neurotoxicity in the 6-hydroxydopamine model of Parkinson's disease.J Ethnopharmacol. 2009 Nov 12;126(2):361-5. doi: 10.1016/j.jep.2009.08.023. Epub 2009 Aug 22. J Ethnopharmacol. 2009. PMID: 19703534
-
Neuroprotective effect of D-psicose on 6-hydroxydopamine-induced apoptosis in rat pheochromocytoma (PC12) cells.J Biosci Bioeng. 2005 Nov;100(5):511-6. doi: 10.1263/jbb.100.511. J Biosci Bioeng. 2005. PMID: 16384789
-
Protective Effect of Quercetin against Oxidative Stress-Induced Cytotoxicity in Rat Pheochromocytoma (PC-12) Cells.Molecules. 2017 Jul 6;22(7):1122. doi: 10.3390/molecules22071122. Molecules. 2017. PMID: 28684704 Free PMC article.
Cited by
-
Effect of bovine milk-derived peptide on SNAP-25 of the neurotransmitter system in treating the sialorrhoea in chronic neurological diseases.Food Sci Biotechnol. 2025 Mar 29;34(11):2601-2610. doi: 10.1007/s10068-025-01872-5. eCollection 2025 Jul. Food Sci Biotechnol. 2025. PMID: 40492044
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

