1-(4-Phenoxybenzyl) 5-Aminouracil Derivatives and Their Analogues - Novel Inhibitors of Human Adenovirus Replication
- PMID: 30116616
- PMCID: PMC6087815
1-(4-Phenoxybenzyl) 5-Aminouracil Derivatives and Their Analogues - Novel Inhibitors of Human Adenovirus Replication
Abstract
Adenovirus infections are characterized by widespread distribution. The lack of causal therapy, which is effective in treating this group of diseases, explains the need for new therapeutic drugs. Notably, anti-adenoviral activity of [4-(phenoxy)benzyl]-5-(phenylamino)-6-azauracil, 1-[4-(phenoxy)benzyl]-5-(morpholino) uracil, 1-[4-(4-chlorophenoxy)benzyl]-5-(morpholino) uracil, and 1-[4-(4-fluorophenoxy)-benzyl]-5-(morpholino) uracil was observed.
Keywords: 5-aminouracil derivatives; Human adenovirus; adenovirus replication; inhibitors of adenovirus replication.
Figures

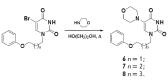
Similar articles
-
Inhibition of uridine phosphorylase. Synthesis and structure-activity relationships of aryl-substituted 1-((2-hydroxyethoxy)methyl)-5-(3-phenoxybenzyl)uracil.J Med Chem. 1997 Apr 11;40(8):1179-85. doi: 10.1021/jm960688j. J Med Chem. 1997. PMID: 9111291
-
Anticoccidial derivatives of 6-azauracil. 1. Enhancement of activity by benzylation of nitrogen-1. Observations on the design of nucleotide analogues in chemotherapy.J Med Chem. 1977 Apr;20(4):475-83. doi: 10.1021/jm00214a003. J Med Chem. 1977. PMID: 576621
-
Synthesis of 1-benzyl-3-(3,5-dimethylbenzyl)uracil derivatives with potential anti-HIV activity.Antivir Chem Chemother. 2011 Oct 7;22(2):57-65. doi: 10.3851/IMP1844. Antivir Chem Chemother. 2011. PMID: 21984685
-
Synthesis, reactivity, and biological activity of 5-aminouracil and its derivatives.Mol Divers. 2016 Feb;20(1):153-83. doi: 10.1007/s11030-015-9595-1. Epub 2015 Apr 30. Mol Divers. 2016. PMID: 25926388 Review.
-
In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications.Eur J Med Chem. 2015 Jun 5;97:582-611. doi: 10.1016/j.ejmech.2014.10.008. Epub 2014 Oct 5. Eur J Med Chem. 2015. PMID: 25306174 Review.
References
-
- Gupta P., Tobias J.D., Goyal S., Hervie P., Harris J.B., Sadot E., Noviski N.. J. Intensive Care Med. 2011;26(4):267–272. - PubMed
-
- Gonzalez-Lopez J.J., Morcillo-Laiz R., Munoz-Negrete F.J.. Arch. Soc. Esp. Oftalmol. 2013;88(3):108–115. - PubMed
-
- Eckardt A.J., Baumgart D.C.. Recent Pat Antiinfect Drug Discov. 2011;6(1):54–63. - PubMed
LinkOut - more resources
Full Text Sources
