Transpalpebral Electrical Stimulation as a Novel Therapeutic Approach to Decrease Intraocular Pressure for Open-Angle Glaucoma: A Pilot Study
- PMID: 30116627
- PMCID: PMC6079529
- DOI: 10.1155/2018/2930519
Transpalpebral Electrical Stimulation as a Novel Therapeutic Approach to Decrease Intraocular Pressure for Open-Angle Glaucoma: A Pilot Study
Abstract
Purpose: To determine the effect on intraocular pressure of transpalpebral specific exogenous voltages in a cohort of open-angle glaucoma patients.
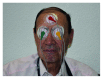
Methods: This is a prospective, comparative, and experimental pilot study. The electrical stimuli applied consisted of 10 Hz, biphasic, nonrectangular current pulses (100 μA) delivered from an isolated constant current stimulator. At intake, baseline IOP measurements were obtained from each eye. The measurement was repeated before and after microstimulation until the end of the treatment.
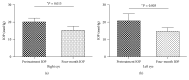
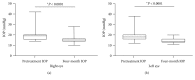
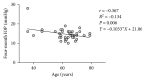
Results: Seventy-eight eyes of 46 patients diagnosed with POAG were studied: 58 eyes with maximum tolerated medical treatment and 20 eyes without treatment (naïve). The mean baseline IOP on the treated POAG group was 19.25 mmHg ± 4.71. Baseline IOP on the naïve group was 20.38 mmHg ± 3.28. At the four-month follow-up visit, the mean IOP value on the treatment group was 14.41 mmHg ± 2.06 (P < 0.0001). The obtained mean IOP measurement on the treatment-naïve group was 15.29 mmHg ± 2.28 (P < 0.0001).
Conclusions: The hypotensive response obtained using transpalpebral electrical stimulation on POAG patients, both on treatment-naïve patients and on patients receiving maximum tolerable treatment, was statistically significant when comparing basal IOP measurements to those obtained at the four-month follow-up visit.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

