Combinations of four or more CpGs methylation present equivalent predictive value for MGMT expression and temozolomide therapeutic prognosis in gliomas
- PMID: 30117294
- PMCID: PMC6488893
- DOI: 10.1111/cns.13040
Combinations of four or more CpGs methylation present equivalent predictive value for MGMT expression and temozolomide therapeutic prognosis in gliomas
Abstract
Aims: The pyrosequencing (PSQ) has been regarded as the gold standard for MGMT promoter methylation testing in gliomas. However, various CpG combinations are currently used in clinical practice. We aimed to clarify how and how many CpGs combined is robust enough to predict MGMT mRNA expression and therapeutic prognosis of patients.
Methods: Total 223 patients with WHO III/IV gliomas were enrolled from Chinese Glioma Genome Atlas, including two independent cohorts, the eight-site cohort (with CpGs 75-82 tested) and the seven-site cohort (with CpGs 72-78 tested). Spearman's correlation and ROC curves were employed to investigate the value of different CpG combinations on predicting MGMT mRNA expression. The ROC curves and Kaplan-Meier steps were performed to compare the TMZ therapeutic prognostic values of different CpG combinations.
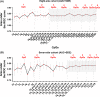
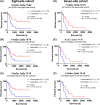
Results: The methylation level of all individual CpG and CpG combinations for the eleven CpGs (CpGs 72-82), significantly correlated to MGMT mRNA expression (Spearman, all P < 0.0001), could effectively predict the mRNA expression (AUC, 0.86-0.91 in the eight-site cohort, 0.83-0.90 in the seven-site cohort). Moreover, the correlation coefficients and the predictive values presented equivalent when four or more CpGs combinedly used (AUC, 0.88-0.90 in the eight-site cohort, 0.87-0.88 in the seven-site cohort). Finally, similar results were also observed when using selected CpG combinations to predict therapeutic prognosis of patients.
Conclusions: Four-CpG combinations of pyrosequencing are sufficient for evaluating the methylation status of MGMT and predicting therapeutic prognosis in gliomas.
Keywords: M GMT; CpGs; glioma; pyrosequencing; temozolomide.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
-
- Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4(4):296‐307. - PubMed
-
- Esteller M, Garcia‐Foncillas J, Andion E, et al. Inactivation of the DNA‐repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350‐1354. - PubMed
-
- Watts GS, Pieper RO, Costello JF, Peng YM, Dalton WS, Futscher BW. Methylation of discrete regions of the O6‐methylguanine DNA methyltransferase (MGMT) CpG island is associated with heterochromatinization of the MGMT transcription start site and silencing of the gene. Mol Cell Biol. 1997;17(9):5612‐5619. - PMC - PubMed
-
- Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O6‐methylguanine‐DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871‐1874. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997‐1003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

