Bradykinin-mediated Ca2+ signalling regulates cell growth and mobility in human cardiac c-Kit+ progenitor cells
- PMID: 30117680
- PMCID: PMC6156395
- DOI: 10.1111/jcmm.13706
Bradykinin-mediated Ca2+ signalling regulates cell growth and mobility in human cardiac c-Kit+ progenitor cells
Abstract
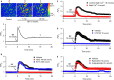
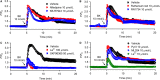
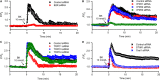
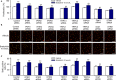
Our recent study showed that bradykinin increases cell cycling progression and migration of human cardiac c-Kit+ progenitor cells by activating pAkt and pERK1/2 signals. This study investigated whether bradykinin-mediated Ca2+ signalling participates in regulating cellular functions in cultured human cardiac c-Kit+ progenitor cells using laser scanning confocal microscopy and biochemical approaches. It was found that bradykinin increased cytosolic free Ca2+ ( ) by triggering a transient Ca2+ release from ER IP3Rs followed by sustained Ca2+ influx through store-operated Ca2+ entry (SOCE) channel. Blockade of B2 receptor with HOE140 or IP3Rs with araguspongin B or silencing IP3R3 with siRNA abolished both Ca2+ release and Ca2+ influx. It is interesting to note that the bradykinin-induced cell cycle progression and migration were not observed in cells with siRNA-silenced IP3R3 or the SOCE component TRPC1, Orai1 or STIM1. Also the bradykinin-induced increase in pAkt and pERK1/2 as well as cyclin D1 was reduced in these cells. These results demonstrate for the first time that bradykinin-mediated increase in free via ER-IP3R3 Ca2+ release followed by Ca2+ influx through SOCE channel plays a crucial role in regulating cell growth and migration via activating pAkt, pERK1/2 and cyclin D1 in human cardiac c-Kit+ progenitor cells.
Keywords: bradykinin; cell cycle progression; human cardiac c-Kit+ progenitor cells; inositol 1,4,5-triphosphate receptor; migration; store-operated Ca2+ entry.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


Similar articles
-
Roles of store-operated Ca2+ channels in regulating cell cycling and migration of human cardiac c-kit+ progenitor cells.Am J Physiol Heart Circ Physiol. 2015 Nov 15;309(10):H1772-81. doi: 10.1152/ajpheart.00260.2015. Epub 2015 Oct 9. Am J Physiol Heart Circ Physiol. 2015. PMID: 26453325
-
Bradykinin regulates cell growth and migration in cultured human cardiac c-Kit+ progenitor cells.Oncotarget. 2017 Feb 14;8(7):10822-10835. doi: 10.18632/oncotarget.14609. Oncotarget. 2017. PMID: 28099911 Free PMC article.
-
STIM1 at the plasma membrane as a new target in progressive chronic lymphocytic leukemia.J Immunother Cancer. 2019 Apr 23;7(1):111. doi: 10.1186/s40425-019-0591-3. J Immunother Cancer. 2019. PMID: 31014395 Free PMC article.
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
-
Tuning store-operated calcium entry to modulate Ca2+-dependent physiological processes.Biochim Biophys Acta Mol Cell Res. 2019 Jul;1866(7):1037-1045. doi: 10.1016/j.bbamcr.2018.11.018. Epub 2018 Dec 3. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30521873 Review.
Cited by
-
Deletion of endothelial IGFBP5 protects against ischaemic hindlimb injury by promoting angiogenesis.Clin Transl Med. 2024 Jun;14(6):e1725. doi: 10.1002/ctm2.1725. Clin Transl Med. 2024. PMID: 38886900 Free PMC article.
-
NEDD4L is a promoter for angiogenesis and cell proliferation in human umbilical vein endothelial cells.J Cell Mol Med. 2024 Apr;28(8):1-11. doi: 10.1111/jcmm.18233. J Cell Mol Med. 2024. PMID: 38526036 Free PMC article.
-
Concanavalin A promotes angiogenesis and proliferation in endothelial cells through the Akt/ERK/Cyclin D1 axis.Pharm Biol. 2022 Dec;60(1):65-74. doi: 10.1080/13880209.2021.2013259. Pharm Biol. 2022. PMID: 34913414 Free PMC article.
-
NMDARs antagonist MK801 suppresses LPS-induced apoptosis and mitochondrial dysfunction by regulating subunits of NMDARs via the CaM/CaMKII/ERK pathway.Cell Death Discov. 2023 Feb 11;9(1):59. doi: 10.1038/s41420-023-01362-9. Cell Death Discov. 2023. PMID: 36774369 Free PMC article.
-
Pretreatment of cardiac progenitor cells with bradykinin attenuates H2O2-induced cell apoptosis and improves cardiac function in rats by regulating autophagy.Stem Cell Res Ther. 2021 Aug 5;12(1):437. doi: 10.1186/s13287-021-02503-6. Stem Cell Res Ther. 2021. PMID: 34353364 Free PMC article.
References
-
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763‐776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

