MAPLE (modular automated platform for large-scale experiments), a robot for integrated organism-handling and phenotyping
- PMID: 30117804
- PMCID: PMC6193762
- DOI: 10.7554/eLife.37166
MAPLE (modular automated platform for large-scale experiments), a robot for integrated organism-handling and phenotyping
Abstract
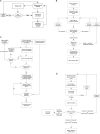
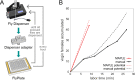
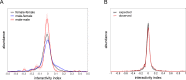
Lab organisms are valuable in part because of large-scale experiments like screens, but performing such experiments over long time periods by hand is arduous and error-prone. Organism-handling robots could revolutionize large-scale experiments in the way that liquid-handling robots accelerated molecular biology. We developed a modular automated platform for large-scale experiments (MAPLE), an organism-handling robot capable of conducting lab tasks and experiments, and then deployed it to conduct common experiments in Saccharomyces cerevisiae, Caenorhabditis elegans, Physarum polycephalum, Bombus impatiens, and Drosophila melanogaster. Focusing on fruit flies, we developed a suite of experimental modules that permitted the automated collection of virgin females and execution of an intricate and laborious social behavior experiment. We discovered that (1) pairs of flies exhibit persistent idiosyncrasies in social behavior, which (2) require olfaction and vision, and (3) social interaction network structure is stable over days. These diverse examples demonstrate MAPLE's versatility for automating experimental biology.
Keywords: C. elegans; D. melanogaster; S. cerevisiae; automation; bombus impatiens; culturing; experimental robot; high throughput phenotyping; neuroscience; physarum polycephalum.
© 2018, Alisch et al.
Conflict of interest statement
TA, JC, AK, DZ No competing interests declared, Bd Benjamin de Bivort is on the scientific advisory board of FlySorter, LLC.
Figures












References
-
- Billeter J-C, Levine JD. The role of cVA and the odorant binding protein lush in social and sexual behavior in Drosophila Melanogaster. Frontiers in Ecology and Evolution. 2015;3 doi: 10.3389/fevo.2015.00075. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

