The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity
- PMID: 30119997
- PMCID: PMC6104739
- DOI: 10.1016/j.immuni.2018.07.010
The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity
Erratum in
-
The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity.Immunity. 2019 Jun 18;50(6):1542. doi: 10.1016/j.immuni.2019.05.024. Immunity. 2019. PMID: 31216463 Free PMC article. No abstract available.
Abstract
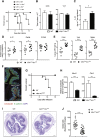
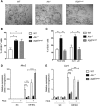
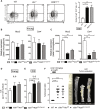
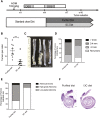
The epithelium and immune compartment in the intestine are constantly exposed to a fluctuating external environment. Defective communication between these compartments at this barrier surface underlies susceptibility to infections and chronic inflammation. Environmental factors play a significant, but mechanistically poorly understood, role in intestinal homeostasis. We found that regeneration of intestinal epithelial cells (IECs) upon injury through infection or chemical insults was profoundly influenced by the environmental sensor aryl hydrocarbon receptor (AHR). IEC-specific deletion of Ahr resulted in failure to control C. rodentium infection due to unrestricted intestinal stem cell (ISC) proliferation and impaired differentiation, culminating in malignant transformation. AHR activation by dietary ligands restored barrier homeostasis, protected the stem cell niche, and prevented tumorigenesis via transcriptional regulation of of Rnf43 and Znrf3, E3 ubiquitin ligases that inhibit Wnt-β-catenin signaling and restrict ISC proliferation. Thus, activation of the AHR pathway in IECs guards the stem cell niche to maintain intestinal barrier integrity.
Keywords: AHR; IBD; Wnt-β-catenin; colon cancer; crypt stem cell; diet; goblet cells; gut barrier; indole-3-carbinol; inflammation; intestinal epithelial cell.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Effects of high-fat diet and intestinal aryl hydrocarbon receptor deletion on colon carcinogenesis.Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G451-G463. doi: 10.1152/ajpgi.00268.2019. Epub 2020 Jan 6. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 31905023 Free PMC article.
-
AhR activation defends gut barrier integrity against damage occurring in obesity.Mol Metab. 2020 Sep;39:101007. doi: 10.1016/j.molmet.2020.101007. Epub 2020 Apr 28. Mol Metab. 2020. PMID: 32360426 Free PMC article.
-
Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles.Science. 2011 Dec 16;334(6062):1561-5. doi: 10.1126/science.1214914. Epub 2011 Oct 27. Science. 2011. PMID: 22033518
-
Aryl hydrocarbon receptor (AhR) and pregnane X receptor (PXR) play both distinct and common roles in the regulation of colon homeostasis and intestinal carcinogenesis.Biochem Pharmacol. 2023 Oct;216:115797. doi: 10.1016/j.bcp.2023.115797. Epub 2023 Sep 9. Biochem Pharmacol. 2023. PMID: 37696457 Review.
-
Regulation of Intestinal Stem Cell Stemness by the Aryl Hydrocarbon Receptor and Its Ligands.Front Immunol. 2021 Mar 10;12:638725. doi: 10.3389/fimmu.2021.638725. eCollection 2021. Front Immunol. 2021. PMID: 33777031 Free PMC article. Review.
Cited by
-
Modulation of Immune Responses by Nutritional Ligands of Aryl Hydrocarbon Receptor.Front Immunol. 2021 May 20;12:645168. doi: 10.3389/fimmu.2021.645168. eCollection 2021. Front Immunol. 2021. PMID: 34093534 Free PMC article. Review.
-
Localization and movement of Tregs in gastrointestinal tract: a systematic review.Inflamm Regen. 2022 Nov 3;42(1):47. doi: 10.1186/s41232-022-00232-8. Inflamm Regen. 2022. PMID: 36329556 Free PMC article. Review.
-
Dysregulation of the Kynurenine Pathway, Cytokine Expression Pattern, and Proteomics Profile Link to Symptomology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).Mol Neurobiol. 2024 Jul;61(7):3771-3787. doi: 10.1007/s12035-023-03784-z. Epub 2023 Nov 28. Mol Neurobiol. 2024. PMID: 38015302
-
Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway.Nat Commun. 2019 Jan 9;10(1):89. doi: 10.1038/s41467-018-07859-7. Nat Commun. 2019. PMID: 30626868 Free PMC article.
-
Intestinal epithelial cell metabolism at the interface of microbial dysbiosis and tissue injury.Mucosal Immunol. 2022 Apr;15(4):595-604. doi: 10.1038/s41385-022-00514-x. Epub 2022 May 9. Mucosal Immunol. 2022. PMID: 35534699 Free PMC article. Review.
References
-
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

