Dysregulated Calcium Homeostasis in Cystic Fibrosis Neutrophils Leads to Deficient Antimicrobial Responses
- PMID: 30120123
- PMCID: PMC6143431
- DOI: 10.4049/jimmunol.1800076
Dysregulated Calcium Homeostasis in Cystic Fibrosis Neutrophils Leads to Deficient Antimicrobial Responses
Abstract
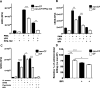
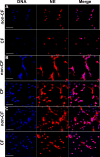
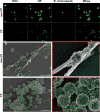
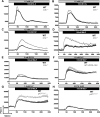
Cystic fibrosis (CF), one of the most common human genetic diseases worldwide, is caused by a defect in the CF transmembrane conductance regulator (CFTR). Patients with CF are highly susceptible to infections caused by opportunistic pathogens (including Burkholderia cenocepacia), which induce excessive lung inflammation and lead to the eventual loss of pulmonary function. Abundant neutrophil recruitment into the lung is a key characteristic of bacterial infections in CF patients. In response to infection, inflammatory neutrophils release reactive oxygen species and toxic proteins, leading to aggravated lung tissue damage in patients with CF. The present study shows a defect in reactive oxygen species production by mouse Cftr-/- , human F508del-CFTR, and CF neutrophils; this results in reduced antimicrobial activity against B. cenocepacia Furthermore, dysregulated Ca2+ homeostasis led to increased intracellular concentrations of Ca2+ that correlated with significantly diminished NADPH oxidase response and impaired secretion of neutrophil extracellular traps in human CF neutrophils. Functionally deficient human CF neutrophils recovered their antimicrobial killing capacity following treatment with pharmacological inhibitors of Ca2+ channels and CFTR channel potentiators. Our findings suggest that regulation of neutrophil Ca2+ homeostasis (via CFTR potentiation or by the regulation of Ca2+ channels) can be used as a new therapeutic approach for reestablishing immune function in patients with CF.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures



References
-
- CFF. Patient registry annual data report 2015
-
- Childers M, Eckel G, Himmel A, Caldwell J. A new model of cystic fibrosis pathology: lack of transport of glutathione and its thiocyanate conjugates. Med Hypotheses. 2007;68:101–112. - PubMed
-
- Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

