Permanent Chemotherapy-Induced Alopecia in Patients with Breast Cancer: A 3-Year Prospective Cohort Study
- PMID: 30120165
- PMCID: PMC6519756
- DOI: 10.1634/theoncologist.2018-0184
Permanent Chemotherapy-Induced Alopecia in Patients with Breast Cancer: A 3-Year Prospective Cohort Study
Abstract
Background: Although chemotherapy-induced alopecia (CIA) is considered temporary, some patients report persistent alopecia several years after chemotherapy. There is, however, a paucity of long-term prospective data on the incidence and impact of permanent CIA (PCIA). The objective of our study was to estimate the long-term incidence of PCIA in a cohort of patients with breast cancer whose hair volume and density were measured prior to chemotherapy and who were followed for 3 years after chemotherapy.
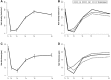
Materials and methods: Prospective cohort study of consecutive patients ≥18 years of age with postoperative diagnosis of stage I-III breast cancer expected to receive adjuvant chemotherapy at the outpatient breast cancer clinic at the Samsung Medical Center in Seoul, Korea, from February 2012 to July 2013 (n = 61). Objective hair density and thickness were measured using a noninvasive bioengineering device.
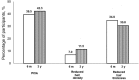
Results: The proportion of participants who had PCIA at 6 months and 3 years was 39.5% and 42.3%, respectively. PCIA was characterized in most patients by incomplete hair regrowth. Patients who received a taxane-based regimen were more likely to experience PCIA compared with patients with other types of chemotherapy. At a 3-year follow-up, hair thinning was the most common problem reported by study participants (75.0%), followed by reduced hair volume (53.9%), hair loss (34.6%), and gray hair (34.6%).
Conclusion: PCIA is a common adverse event of breast cancer adjuvant cytotoxic chemotherapy. Clinicians should be aware of this distressing adverse event and develop supportive care strategies to counsel patients and minimize its impact on quality of life.
Implications for practice: Knowledge of permanent chemotherapy-induced alopecia, an under-reported adverse event, should lead to optimized pretherapy counseling, anticipatory coping techniques, and potential therapeutic strategies for this sequela of treatment.
Keywords: Alopecia; Breast neoplasm; Chemotherapy; Cohort.
© AlphaMed Press 2018.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- Yabroff KR, Lawrence WF, Clauser S et al. Burden of illness in cancer survivors: Findings from a population‐based national sample. J Natl Cancer Instit 2004;96:1322–1330. - PubMed
-
- Nissen MJ, Swenson KK, Ritz LJ et al. Quality of life after breast carcinoma surgery: A comparison of three surgical procedures. Cancer 2001;91:1238–1246. - PubMed
-
- Zielinski C, Beslija S, Mrsic‐Krmpotic Z et al. Gemcitabine, epirubicin, and paclitaxel versus fluorouracil, epirubicin, and cyclophosphamide as first‐line chemotherapy in metastatic breast cancer: A Central European Cooperative Oncology Group international, multicenter, prospective, randomized phase III trial. J Clin Oncol 2005;23:1401–1408. - PubMed
-
- Kim IR, Cho JH, Choi EK et al. Perception, attitudes, preparedness and experience of chemotherapy‐induced alopecia among breast cancer patients: A qualitative study. Asian Pac J Cancer Prev 2012;13:1383–1388. - PubMed
-
- McGarvey EL, Baum LD, Pinkerton RC et al. Psychological sequelae and alopecia among women with cancer. Cancer Pract 2001;9:283–289. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

