Glucose Uptake via STP Transporters Inhibits in Vitro Pollen Tube Growth in a HEXOKINASE1-Dependent Manner in Arabidopsis thaliana
- PMID: 30120167
- PMCID: PMC6181011
- DOI: 10.1105/tpc.18.00356
Glucose Uptake via STP Transporters Inhibits in Vitro Pollen Tube Growth in a HEXOKINASE1-Dependent Manner in Arabidopsis thaliana
Abstract
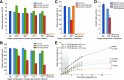
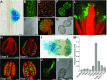
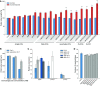
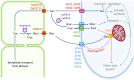
Pollen tube growth requires a high amount of metabolic energy and precise targeting toward the ovules. Sugars, especially glucose, can serve as nutrients and as signaling molecules. Unexpectedly, in vitro assays revealed an inhibitory effect of glucose on pollen tube elongation, contradicting the hypothesis that monosaccharide uptake is a source of nutrition for growing pollen tubes. Measurements with Förster resonance energy transfer-based nanosensors revealed that glucose is taken up into pollen tubes and that the intracellular concentration is in the low micromolar range. Pollen tubes of stp4-6-8-9-10-11 sextuple knockout plants generated by crossings and CRISPR/Cas9 showed only a weak response to glucose, indicating that glucose uptake into pollen tubes is mediated mainly by these six monosaccharide transporters of the SUGAR TRANSPORT PROTEIN (STP) family. Analyses of HEXOKINASE1 (HXK1) showed a strong expression of this gene in pollen. Together with the glucose insensitivity and altered semi-in vivo growth rate of pollen tubes from hxk1 knockout lines, this strongly suggests that glucose is an important signaling molecule for pollen tubes, is taken up by STPs, and detected by HXK1. Equimolar amounts of fructose abolish the inhibitory effect of glucose indicating that only an excess of glucose is interpreted as a signal. This provides a possible model for the discrimination of signaling and nutritional sugars.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures








References
-
- Abel S., Theologis A. (1994). Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J. 5: 421–427. - PubMed
-
- Aguilera-Alvarado G.P., Sánchez-Nieto S. (2017). Plant hexokinases are multifaceted proteins. Plant Cell Physiol. 58: 1151–1160. - PubMed
-
- Aki T., Konishi M., Kikuchi T., Fujimori T., Yoneyama T., Yanagisawa S. (2007). Distinct modulations of the hexokinase1-mediated glucose response and hexokinase1-independent processes by HYS1/CPR5 in Arabidopsis. J. Exp. Bot. 58: 3239–3248. - PubMed
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

