Smooth tracking of visual targets distinguishes lucid REM sleep dreaming and waking perception from imagination
- PMID: 30120229
- PMCID: PMC6098118
- DOI: 10.1038/s41467-018-05547-0
Smooth tracking of visual targets distinguishes lucid REM sleep dreaming and waking perception from imagination
Abstract
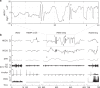
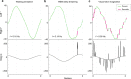
Humans are typically unable to engage in sustained smooth pursuit for imagined objects. However, it is unknown to what extent smooth tracking occurs for visual imagery during REM sleep dreaming. Here we examine smooth pursuit eye movements during tracking of a slow-moving visual target during lucid dreams in REM sleep. Highly similar smooth pursuit tracking was observed during both waking perception and lucid REM sleep dreaming, in contrast to the characteristically saccadic tracking observed during visuomotor imagination. Our findings suggest that, in this respect, the visual imagery that occurs during REM sleep is more similar to perception than imagination. The data also show that the neural circuitry of smooth pursuit can be driven by a visual percept in the absence of retinal stimulation and that specific voluntary shifts in the direction of experienced gaze within REM sleep dreams are accompanied by corresponding rotations of the physical eyes.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
REM sleep eye movement counts correlate with visual imagery in dreaming: a pilot study.Psychophysiology. 1997 May;34(3):377-81. doi: 10.1111/j.1469-8986.1997.tb02408.x. Psychophysiology. 1997. PMID: 9175452
-
Terror and bliss? Commonalities and distinctions between sleep paralysis, lucid dreaming, and their associations with waking life experiences.J Sleep Res. 2017 Feb;26(1):38-47. doi: 10.1111/jsr.12441. Epub 2016 Jul 27. J Sleep Res. 2017. PMID: 27460633 Free PMC article.
-
Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming.Sleep. 2009 Sep;32(9):1191-200. doi: 10.1093/sleep/32.9.1191. Sleep. 2009. PMID: 19750924 Free PMC article.
-
The right hemisphere in imagery, hypnosis, rapid eye movement sleep and dreaming. Empirical studies and tentative conclusions.J Nerv Ment Dis. 1988 Jun;176(6):323-31. doi: 10.1097/00005053-198806000-00001. J Nerv Ment Dis. 1988. PMID: 3286818 Review.
-
REM sleep and dreaming: towards a theory of protoconsciousness.Nat Rev Neurosci. 2009 Nov;10(11):803-13. doi: 10.1038/nrn2716. Epub 2009 Oct 1. Nat Rev Neurosci. 2009. PMID: 19794431 Review.
Cited by
-
Dreaming conundrum.J Sleep Res. 2025 Apr;34(2):e14338. doi: 10.1111/jsr.14338. Epub 2024 Oct 3. J Sleep Res. 2025. PMID: 39360736 Free PMC article. Review.
-
Offline perception: an introduction.Philos Trans R Soc Lond B Biol Sci. 2021 Feb;376(1817):20190686. doi: 10.1098/rstb.2019.0686. Epub 2020 Dec 14. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 33308069 Free PMC article.
-
Frequent lucid dreaming associated with increased functional connectivity between frontopolar cortex and temporoparietal association areas.Sci Rep. 2018 Dec 12;8(1):17798. doi: 10.1038/s41598-018-36190-w. Sci Rep. 2018. PMID: 30542052 Free PMC article.
-
Wake Up, Work on Dreams, Back to Bed and Lucid Dream: A Sleep Laboratory Study.Front Psychol. 2020 Jun 26;11:1383. doi: 10.3389/fpsyg.2020.01383. eCollection 2020. Front Psychol. 2020. PMID: 32670163 Free PMC article.
-
Modulating dream experience: Noninvasive brain stimulation over the sensorimotor cortex reduces dream movement.Sci Rep. 2020 Apr 21;10(1):6735. doi: 10.1038/s41598-020-63479-6. Sci Rep. 2020. PMID: 32317714 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

