Axoneme polyglutamylation regulated by Joubert syndrome protein ARL13B controls ciliary targeting of signaling molecules
- PMID: 30120249
- PMCID: PMC6098020
- DOI: 10.1038/s41467-018-05867-1
Axoneme polyglutamylation regulated by Joubert syndrome protein ARL13B controls ciliary targeting of signaling molecules
Abstract
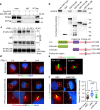
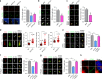
Tubulin polyglutamylation is a predominant axonemal post-translational modification. However, if and how axoneme polyglutamylation is essential for primary cilia and contribute to ciliopathies are unknown. Here, we report that Joubert syndrome protein ARL13B controls axoneme polyglutamylation, which is marginally required for cilia stability but essential for cilia signaling. ARL13B interacts with RAB11 effector FIP5 to promote cilia import of glutamylase TTLL5 and TTLL6. Hypoglutamylation caused by a deficient ARL13B-RAB11-FIP5 trafficking pathway shows no effect on ciliogenesis, but promotes cilia disassembly and, importantly, impairs cilia signaling by disrupting the proper anchoring of sensory receptors and trafficking of signaling molecules. Remarkably, depletion of deglutamylase CCP5, the predominant cilia deglutamylase, effectively restores hypoglutamylation-induced cilia defects. Our study reveals a paradigm that tubulin polyglutamylation is a major contributor for cilia signaling and suggests a potential therapeutic strategy by targeting polyglutamylation machinery to promote ciliary targeting of signaling machineries and correct signaling defects in ciliopathies.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
The Joubert syndrome protein ARL13B binds tubulin to maintain uniform distribution of proteins along the ciliary membrane.J Cell Sci. 2018 May 4;131(9):jcs212324. doi: 10.1242/jcs.212324. J Cell Sci. 2018. PMID: 29592971 Free PMC article.
-
Regulation of ciliary retrograde protein trafficking by the Joubert syndrome proteins ARL13B and INPP5E.J Cell Sci. 2017 Feb 1;130(3):563-576. doi: 10.1242/jcs.197004. Epub 2016 Dec 7. J Cell Sci. 2017. PMID: 27927754
-
A function for the Joubert syndrome protein Arl13b in ciliary membrane extension and ciliary length regulation.Dev Biol. 2015 Jan 15;397(2):225-36. doi: 10.1016/j.ydbio.2014.11.009. Epub 2014 Nov 20. Dev Biol. 2015. PMID: 25448689
-
Ciliopathies and the Kidney: A Review.Am J Kidney Dis. 2021 Mar;77(3):410-419. doi: 10.1053/j.ajkd.2020.08.012. Epub 2020 Oct 9. Am J Kidney Dis. 2021. PMID: 33039432 Review.
-
The Joubert-Meckel-Nephronophthisis Spectrum of Ciliopathies.Annu Rev Genomics Hum Genet. 2022 Aug 31;23:301-329. doi: 10.1146/annurev-genom-121321-093528. Epub 2022 Jun 2. Annu Rev Genomics Hum Genet. 2022. PMID: 35655331 Free PMC article. Review.
Cited by
-
The Emerging Roles of Axonemal Glutamylation in Regulation of Cilia Architecture and Functions.Front Cell Dev Biol. 2021 Mar 4;9:622302. doi: 10.3389/fcell.2021.622302. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748109 Free PMC article. Review.
-
Proximity Mapping of CCP6 Reveals Its Association with Centrosome Organization and Cilium Assembly.Int J Mol Sci. 2023 Jan 9;24(2):1273. doi: 10.3390/ijms24021273. Int J Mol Sci. 2023. PMID: 36674791 Free PMC article.
-
Tubulin Post-translational Modifications: Potential Therapeutic Approaches to Heart Failure.Front Cell Dev Biol. 2022 Apr 12;10:872058. doi: 10.3389/fcell.2022.872058. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493101 Free PMC article. Review.
-
Transiently formed nucleus-to-cilium microtubule arrays mediate senescence initiation in a KIFC3-dependent manner.Nat Commun. 2024 Sep 12;15(1):7977. doi: 10.1038/s41467-024-52363-w. Nat Commun. 2024. PMID: 39266565 Free PMC article.
-
TTC30A and TTC30B Redundancy Protects IFT Complex B Integrity and Its Pivotal Role in Ciliogenesis.Genes (Basel). 2022 Jul 1;13(7):1191. doi: 10.3390/genes13071191. Genes (Basel). 2022. PMID: 35885974 Free PMC article.
References
-
- Audebert S, et al. Developmental regulation of polyglutamylated alpha- and beta-tubulin in mouse brain neurons. J. Cell. Sci. 1994;107(Pt 8):2313–2322. - PubMed
-
- Bedoni. N, et al. Mutations in the polyglutamylase gene TTLL5, expressed in photoreceptor cells and spermatozoa, are associated with cone-rod degeneration and reduced male fertility. Hum. Mol. Genet. 2016;25:4546–4555. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

