T-complex protein 1-ring complex enhances retrograde axonal transport by modulating tau phosphorylation
- PMID: 30120810
- PMCID: PMC6191364
- DOI: 10.1111/tra.12610
T-complex protein 1-ring complex enhances retrograde axonal transport by modulating tau phosphorylation
Abstract
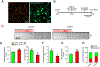
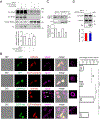
The cytosolic chaperonin T-complex protein (TCP) 1-ring complex (TRiC) has been shown to exert neuroprotective effects on axonal transport through clearance of mutant Huntingtin (mHTT) in Huntington's disease. However, it is presently unknown if TRiC also has any effect on axonal transport in wild-type neurons. Here, we examined how TRiC impacted the retrograde axonal transport of brain-derived neurotrophic factor (BDNF). We found that expression of a single TRiC subunit significantly enhanced axonal transport of BDNF, leading to an increase in instantaneous velocity with a concomitant decrease in pauses for retrograde BDNF transport. The transport enhancing effect by TRiC was dependent on endogenous tau expression because no effect was seen in neurons from tau knockout mice. We showed that TRiC regulated the level of cyclin-dependent kinase 5 (CDK5)/p35 positively, contributing to TRiC-mediated tau phosphorylation (ptau). Expression of a single TRiC subunit increased the level of ptau while downregulation of the TRiC complex decreased ptau. We further demonstrated that TRiC-mediated increase in ptau induced detachment of tau from microtubules. Our study has thus revealed that TRiC-mediated increase in tau phosphorylation impacts retrograde axonal transport.
Keywords: TRiC/CCT chaperonin; axonal transport; hyperphosphorylation; tau.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

