Skeletal muscle interstitial O2 pressures: bridging the gap between the capillary and myocyte
- PMID: 30120845
- PMCID: PMC6379155
- DOI: 10.1111/micc.12497
Skeletal muscle interstitial O2 pressures: bridging the gap between the capillary and myocyte
Abstract
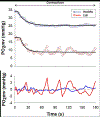
The oxygen transport pathway from air to mitochondria involves a series of transfer steps within closely integrated systems (pulmonary, cardiovascular, and tissue metabolic). Small and finite O2 stores in most mammalian species require exquisitely controlled changes in O2 flux rates to support elevated ATP turnover. This is especially true for the contracting skeletal muscle where O2 requirements may increase two orders of magnitude above rest. This brief review focuses on the mechanistic bases for increased microvascular blood-myocyte O2 flux (V̇O2 ) from rest to contractions. Fick's law dictates that V̇O2 elevations driven by muscle contractions are produced by commensurate changes in driving force (ie, O2 pressure gradients; ΔPO2 ) and/or effective diffusing capacity (DO2 ). While previous evidence indicates that increased DO2 helps modulate contracting muscle O2 flux, up until recently the role of the dynamic ΔPO2 across the capillary wall was unknown. Recent phosphorescence quenching investigations of both microvascular and novel interstitial PO2 kinetics in health have resolved an important step in the O2 cascade between the capillary and myocyte. Specifically, the significant transmural ΔPO2 at rest was sustained (but not increased) during submaximal contractions. This supports the contention that the blood-myocyte interface provides a substantial effective resistance to O2 diffusion and underscores that modulations in erythrocyte hemodynamics and distribution (DO2 ) are crucial to preserve the driving force for O2 flux across the capillary wall (ΔPO2 ) during contractions. Investigation of the O2 transport pathway close to muscle mitochondria is key to identifying disease mechanisms and develop therapeutic approaches to ameliorate dysfunction and exercise intolerance.
Keywords: capillary; diffusion; exercise; microcirculation; oxygen gradients.
© 2018 John Wiley & Sons Ltd.
Figures
Similar articles
-
The role of vascular function on exercise capacity in health and disease.J Physiol. 2021 Feb;599(3):889-910. doi: 10.1113/JP278931. Epub 2020 Mar 3. J Physiol. 2021. PMID: 31977068 Free PMC article. Review.
-
Skeletal muscle interstitial Po2 kinetics during recovery from contractions.J Appl Physiol (1985). 2019 Oct 1;127(4):930-939. doi: 10.1152/japplphysiol.00297.2019. Epub 2019 Aug 1. J Appl Physiol (1985). 2019. PMID: 31369325 Free PMC article.
-
Skeletal muscle microvascular and interstitial PO2 from rest to contractions.J Physiol. 2018 Mar 1;596(5):869-883. doi: 10.1113/JP275170. Epub 2018 Jan 30. J Physiol. 2018. PMID: 29288568 Free PMC article.
-
Transcapillary PO2 gradients in contracting muscles across the fibre type and oxidative continuum.J Physiol. 2020 Aug;598(15):3187-3202. doi: 10.1113/JP279608. Epub 2020 Jun 12. J Physiol. 2020. PMID: 32445225 Free PMC article.
-
Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization.Am J Physiol Heart Circ Physiol. 2015 Nov;309(9):H1419-39. doi: 10.1152/ajpheart.00469.2015. Epub 2015 Aug 28. Am J Physiol Heart Circ Physiol. 2015. PMID: 26320036 Free PMC article. Review.
Cited by
-
Use of H2O2 to Cause Oxidative Stress, the Catalase Issue.Int J Mol Sci. 2020 Nov 30;21(23):9149. doi: 10.3390/ijms21239149. Int J Mol Sci. 2020. PMID: 33266350 Free PMC article.
-
August Krogh: Muscle capillary function and oxygen delivery.Comp Biochem Physiol A Mol Integr Physiol. 2021 Mar;253:110852. doi: 10.1016/j.cbpa.2020.110852. Epub 2020 Nov 24. Comp Biochem Physiol A Mol Integr Physiol. 2021. PMID: 33242636 Free PMC article. Review.
-
Sexual dimorphism in the control of skeletal muscle interstitial Po2 of heart failure rats: effects of dietary nitrate supplementation.J Appl Physiol (1985). 2019 May 1;126(5):1184-1192. doi: 10.1152/japplphysiol.01004.2018. Epub 2019 Mar 7. J Appl Physiol (1985). 2019. PMID: 30844332 Free PMC article.
-
Scaffold-Mediated Immunoengineering as Innovative Strategy for Tendon Regeneration.Cells. 2022 Jan 13;11(2):266. doi: 10.3390/cells11020266. Cells. 2022. PMID: 35053383 Free PMC article. Review.
-
The role of vascular function on exercise capacity in health and disease.J Physiol. 2021 Feb;599(3):889-910. doi: 10.1113/JP278931. Epub 2020 Mar 3. J Physiol. 2021. PMID: 31977068 Free PMC article. Review.
References
-
- Weibel ER. The Pathway for Oxygen: Structure and Function in the Mammalian Respiratory System Harvard University Press; 1984.
-
- Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206(Pt 12):2011–2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

