Use of VHH antibodies for the development of antigen detection test for visceral leishmaniasis
- PMID: 30120856
- PMCID: PMC6220836
- DOI: 10.1111/pim.12584
Use of VHH antibodies for the development of antigen detection test for visceral leishmaniasis
Abstract
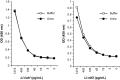
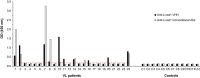
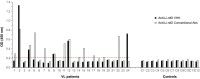
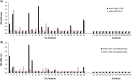
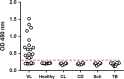
We have recently developed a sensitive and specific urine-based antigen detection ELISA for the diagnosis of visceral leishmaniasis (VL). This assay used rabbit IgG and chicken IgY polyclonal antibodies specific for the Leishmania infantum proteins iron superoxide dismutase 1 (Li-isd1), tryparedoxin1 (Li-txn1) and nuclear transport factor 2 (Li-ntf2). However, polyclonal antibodies have limitations for upscaling and continuous supply. To circumvent these hurdles, we began to develop immortalized monoclonal antibodies. We opted for recombinant camelid VHHs because the technology for their production is well established and they do not have Fc, hence providing less ELISA background noise. We report here an assay development using VHHs specific for Li-isd1 and Li-ntf2. This new assay was specific and had analytical sensitivity of 15-45 pg/mL of urine. The clinical sensitivity was comparable to that obtained with the ELISA assembled with conventional rabbit and chicken antibodies to detect these two antigens. Therefore, similar to our former studies with conventional antibodies, the future inclusion of VHH specific for Li-txn1 and/or other antigens should further increase the sensitivity of the assay. These results confirm that immortalized VHHs can replace conventional antibodies for the development of an accurate and reproducible antigen detection diagnostic test for VL.
© 2018 The Authors. Parasite Immunology Published by John Wiley & Sons Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

