Sacubitril/valsartan: A novel angiotensin receptor-neprilysin inhibitor
- PMID: 30122239
- PMCID: PMC6097164
- DOI: 10.1016/j.ihj.2018.01.002
Sacubitril/valsartan: A novel angiotensin receptor-neprilysin inhibitor
Abstract
Objective: To describe the efficacy, superiority and safety profile of the first-in-class angiotensin receptor-neprilysin inhibitor "Sacubitril/Valsartan" as compared to angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blocker (ARB) in heart failure (HF) patients, reviewing data available from both clinical and pre-clinical studies. Evidences on health care utilization outcomes such as hospitalizations and emergency department visits were also evaluated.
Material (data source): Sources: Medical literature on 'Sacubitril/Valsartan' and 'Angiotensin Receptor-Neprilysin Inhibitor' was identified by searching databases (including, but not limited to, PubMed, Embase and HighWire) for articles published since 1991, bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the companies developing the drug.
Search strategy: We conducted separate searches for each of the interventions of interest. The timeframe for both searches spanned the period from January 1991 to the most recently published data available and focused on PubMed, Embase and HighWire indexed articles. The search strategies included a combination of indexing terms as well as free-text terms included separately in 'Keywords' section. To supplement the above searches and ensure optimal and complete literature retrieval, we performed a manual check of the references of recent relevant reviews and meta-analysis. Searches were last updated on 12th July 2017.
Selection: Studies in patients with hypertension who received sacubitril/valsartan combination drug were included. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well-controlled trials with appropriate statistical methodology was preferred. Relevant pharmacodynamics and pharmacokinetics data was also included.
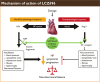
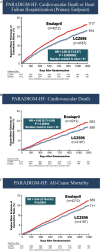
Data evaluation: Many clinical trials have been conducted comparing the efficacy of sacubitril/valsartan with other anti-hypertensives. The trials have shown sacubitril/valsartan to be more effective in improving symptoms and physical limitations, reducing the risk of cardiovascular (CV) death, HF hospitalization, and the overall mortality and morbidity compared to its counterparts.
Conclusion: Effective reduction of blood pressure to accepted goals is the key to reduce the risk of CV events and stroke. Dual inhibition of neprilysin and the angiotensin receptor with sacubitril/valsartan may represent an attractive and serendipitous therapeutic approach for a range of CV diseases, including hypertension and HF, in which vasoconstriction, volume overload and neuro-hormonal activation play a part in pathophysiology. Sacubitril/Valsartan appears to be more efficacious in reducing blood pressure than currently available ACEi and ARBs with a similar safety and tolerability profile. Besides, pleiotropic benefits like HbA1c reduction, better eGFR progression and a greater decrease in blood pressure and serum creatinine levels make this drug a novel addition to the current hypertension armamentarium.
Keywords: Angiotensin converting enzyme inhibitors; Angiotensin receptor blockers; Angiotensin receptor-neprilysin inhibitor; Heart failure; Hypertension; LCZ696; NKindly modify it to: LCZ696atriuretic peptides; Sacubitril; Sacubitril/valsartan; Valsartan.
Copyright © 2018. Published by Elsevier B.V.
Figures

References
-
- Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med. 2006;355(July (3)):251–259. PMID: 16855265 CrossRef. - PubMed
-
- Chaturvedi V., Parakh N., Seth S. Heart failure in India: the INDUS (INDia ukieri study) study. J Pract Cardiovasc Sci. 2016;2:28–35. CrossRef.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

