Prognostic value of soluble ST2 biomarker in heart failure patients with reduced ejection fraction - A multicenter study
- PMID: 30122243
- PMCID: PMC6097172
- DOI: 10.1016/j.ihj.2017.09.010
Prognostic value of soluble ST2 biomarker in heart failure patients with reduced ejection fraction - A multicenter study
Abstract
Objective: To study the prognostic value of soluble Suppression of Tumorigenicity-2 (sST2) in heart failure patients with reduced ejection fraction (HFrEF).
Methods: In this prospective, observational, multicenter study, patients with heart failure (HF) and left ventricular ejection fraction (LVEF) <50% were included. Clinical evaluation and serum levels of sST2 were estimated at five time points during follow up. Study endpoint was the relationship of baseline and serial sST2 concentration in the blood to the composite endpoints of cardiac death and re-hospitalization for worsening of HF during one year follow up period.
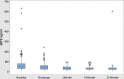
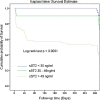
Results: A total of 141 patients were enrolled. The mean age was 60±10.4years. At baseline evaluation, 49.6% patients were in New York Heart Association (NYHA) class III and 36.2% in class IV. Adverse events were observed in 57 patients (40.4%); 25 (17.7%) were re-hospitalized due to worsening of HF and 32 (22.7%) died due to cardiac causes. The median value of baseline sST2 was 46.36ng/ml (IQR 31.30-78.38). sST2 concentration at baseline was significantly higher among patients with adverse events in comparison to patients without adverse events (p=<0.001). Receiver operating characteristic curve (ROC) for baseline sST2 concentration identified 49ng/ml as optimal cut-off value to predict cardiac death and re-hospitalization, with a sensitivity and specificity of 72% and 75%, respectively.
Conclusion: In patients with HFrEF, sST2 concentration at baseline as well as on serial testing was significantly correlated with cardiac death and re-hospitalization for worsening of HF.
Keywords: Biomarker; Heart failure; Prognosis; Serial testing; sST2.
Copyright © 2017. Published by Elsevier B.V.
Figures



References
-
- Mozaffarian D., Benjamin E.J., Go A.S. Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. - PubMed
-
- Chow S.L., Maisel A.S., Anand I. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135 - PubMed
-
- Jaffe A.S., Januzzi J.L. Using biomarkers to guide heart failure therapy. Clin Chem. 2017;63:954–957. - PubMed
-
- Anand I.S., Kempf T., Rector T.S. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the valsartan heart failure trial. Circulation. 2010;122:1387–1395. - PubMed
-
- Masson S., Anand I., Favero C. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from two large randomized clinical trials. Circulation. 2012;125:280–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

