Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting
- PMID: 30122555
- PMCID: PMC6390946
- DOI: 10.1016/j.cmet.2018.07.021
Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting
Abstract
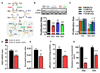
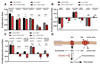
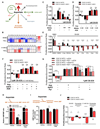
Mitochondrial function is important for aspartate biosynthesis in proliferating cells. Here, we show that mitochondrial aspartate export via the aspartate-glutamate carrier 1 (AGC1) supports cell proliferation and cellular redox homeostasis. Insufficient cytosolic aspartate delivery leads to cell death when TCA cycle carbon is reduced following glutamine withdrawal and/or glutaminase inhibition. Moreover, loss of AGC1 reduces allograft tumor growth that is further compromised by treatment with the glutaminase inhibitor CB-839. Together, these findings argue that mitochondrial aspartate export sustains cell survival in low-glutamine environments and AGC1 inhibition can synergize with glutaminase inhibition to limit tumor growth.
Keywords: AGC1; Aralar; SLC25A12; aspartate-glutamate carrier; cancer metabolism; glutamine metabolism.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
M.G.V.H. discloses that he is a consultant and scientific advisory board member for Agios Pharmaceuticals and Aeglea Biotherapeutics, and T.M. discloses that he is a consultant and scientific advisory board member of Cleara Biotech.
Figures




References
-
- Amoedo ND, Punzi G, Obre E, Lacombe D, De Grassi A, Pierri CL, Rossignol R. AGC1/2, the mitochondrial aspartate-glutamate carriers. Biochim Biophys Acta. 2016;1863:2394–2412. - PubMed
-
- Begum L, Jalil MA, Kobayashi K, Iijima M, Li MX, Yasuda T, Horiuchi M, del Arco A, Satrústegui J, Saheki T. Expression of three mitochondrial solute carriers, citrin, aralar1 and ornithine transporter, in relation to urea cycle in mice. Biochim Biophys Acta. 2002;1574:283–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

