Preclinical safety evaluation of hepatic arterial infusion of oncolytic poxvirus
- PMID: 30122903
- PMCID: PMC6087018
- DOI: 10.2147/DDDT.S171269
Preclinical safety evaluation of hepatic arterial infusion of oncolytic poxvirus
Abstract
Purpose: Oncolytic poxvirus has shown promise in treating various solid tumors, such as liver cancer, and administration of oncolytic poxvirus via the hepatic artery may provide more survival benefits than other routes of administration. However, there is a lack of safety information to guide the application of hepatic arterial infusion (HAI) of oncolytic poxvirus in human studies. To investigate the acute and chronic toxicity of HAI administration of oncolytic poxvirus in animals and provide safety information for future human studies.
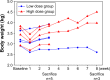
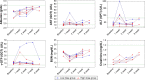
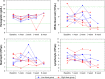
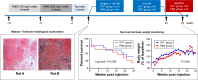
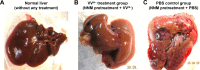
Methods: VVtk-, a vaccinia poxvirus with inactivated thymidine kinase gene, was administered via HAI to rabbits with normal liver function under angiography (1×108 or 1×109 pfu), and rats with N-nitrosomorpholine-induced precancerous liver cirrhosis under open surgery (1×108 pfu). Body weights and survival were monitored and blood samples were collected for hematological and biochemical tests. Distribution of A56 (a specific marker for poxvirus infection) in rabbit organs was evaluated using immunofluorescence assays.
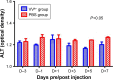
Results: HAI of high doses of VVtk- did not cause any acute or chronic changes in body weight, survival or in biochemical, hematological tests in the 2 animal models, and none of the changes showed dose dependency (in rabbit study), or were influenced by liver cirrhosis (in rat study). A56 was not detected in any of the major rabbit organs.
Conclusion: HAI may provide a safe alternative route of oncolytic poxvirus administration for human studies.
Keywords: hepatocellular carcinoma; liver cirrhosis; oncolytic virus; transhepatic angiography.
Conflict of interest statement
Disclosure Part of the data in this study is going to be submitted for an investigational new drug application by Bionoxx Inc. The authors have no conflicts of interest to declare in this work.
Figures


Similar articles
-
Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial.Lancet Oncol. 2008 Jun;9(6):533-42. doi: 10.1016/S1470-2045(08)70107-4. Epub 2008 May 19. Lancet Oncol. 2008. PMID: 18495536 Clinical Trial.
-
Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF.Mol Ther. 2006 Sep;14(3):361-70. doi: 10.1016/j.ymthe.2006.05.008. Mol Ther. 2006. PMID: 16905462
-
Differential biodistribution of oncolytic poxvirus administered systemically in an autochthonous model of hepatocellular carcinoma.J Gene Med. 2011 Dec;13(12):692-701. doi: 10.1002/jgm.1624. J Gene Med. 2011. PMID: 22028274
-
Poxvirus oncolytic virotherapy.Expert Opin Biol Ther. 2019 Jun;19(6):561-573. doi: 10.1080/14712598.2019.1600669. Epub 2019 Apr 4. Expert Opin Biol Ther. 2019. PMID: 30919708 Review.
-
JX-594, a targeted oncolytic poxvirus for the treatment of cancer.Curr Opin Investig Drugs. 2009 Dec;10(12):1372-82. Curr Opin Investig Drugs. 2009. PMID: 19943208 Review.
Cited by
-
Engineering and Characterization of Oncolytic Vaccinia Virus Expressing Truncated Herpes Simplex Virus Thymidine Kinase.Cancers (Basel). 2020 Jan 17;12(1):228. doi: 10.3390/cancers12010228. Cancers (Basel). 2020. PMID: 31963415 Free PMC article.
-
Recent advances in oncolytic virus therapy for hepatocellular carcinoma.Front Oncol. 2023 Apr 26;13:1172292. doi: 10.3389/fonc.2023.1172292. eCollection 2023. Front Oncol. 2023. PMID: 37182136 Free PMC article. Review.
-
Targeted suicide gene therapy for liver cancer based on ribozyme-mediated RNA replacement through post-transcriptional regulation.Mol Ther Nucleic Acids. 2020 Oct 31;23:154-168. doi: 10.1016/j.omtn.2020.10.036. eCollection 2021 Mar 5. Mol Ther Nucleic Acids. 2020. PMID: 33335800 Free PMC article.
-
The Effects of Oncolytic Pseudorabies Virus Vaccine Strain Inhibited the Growth of Colorectal Cancer HCT-8 Cells In Vitro and In Vivo.Animals (Basel). 2022 Sep 14;12(18):2416. doi: 10.3390/ani12182416. Animals (Basel). 2022. PMID: 36139276 Free PMC article.
-
Characterization of Oncolytic Vaccinia Virus Harboring the Human IFNB1 and CES2 Transgenes.Cancer Res Treat. 2020 Jan;52(1):309-319. doi: 10.4143/crt.2019.161. Epub 2019 Aug 6. Cancer Res Treat. 2020. PMID: 31401821 Free PMC article.
References
-
- Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274–1283. - PubMed
-
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. - PubMed
-
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. - PubMed
-
- Jebar AH, Errington-Mais F, Vile RG, Selby PJ, Melcher AA, Griffin S. Progress in clinical oncolytic virus-based therapy for hepatocellular carcinoma. J Gen Virol. 2015;96(Pt 7):1533–1550. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

