Drug response to PD-1/PD-L1 blockade: based on biomarkers
- PMID: 30122958
- PMCID: PMC6087015
- DOI: 10.2147/OTT.S168313
Drug response to PD-1/PD-L1 blockade: based on biomarkers
Abstract
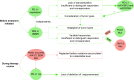
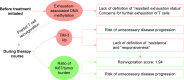
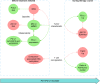
In recent years, immunotherapies targeting programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1) have provided great hopes for patients with cancer. A successful anti-PD-1/PD-L1 therapy includes not only the elimination of immunosuppressive tumor cells but also the rejuvenation of exhausted T cells. Nevertheless, the efficacy of therapy is still low, so that biomarker-driven therapy has attracted more and more attention to identify patients who are likely to benefit from therapy and to reduce unnecessary disease progression. While many studies have focused on characteristics of tumor biopsies, biomarkers linked to T cell exhaustion and rejuvenation have just become new hot spots in drug response studies. However, no biomarker is perfect in drug response prediction currently, so there is an urgent need for other biomarkers to compensate for the deficiency. In this review, we summarize some approved and candidate biomarkers predictive of drug response before and during PD-1/PD-L1 blockade, including those characterizing responsive or suppressive tumor cells and those evaluating the T cell rejuvenation. Overall, we set up a comprehensive network of biomarkers of tumor characteristics and T cell rejuvenation, predicting drug response before and during anti-PD-1/PD-L1 therapies.
Keywords: PD-1; PD-L1; T cell rejuvenation; biomarker; network; tumor.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Salmaninejad A, Khoramshahi V, Azani A, et al. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2018;70(2):73–86. - PubMed
-
- Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74(16):1973–1981. - PubMed
-
- Li H, Yu J, Liu C, et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J Pharmacokinet Pharmacodyn. 2017;44(5):403–414. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

