HIV-1 genotypic drug resistance in patients with virological failure to single-tablet antiretroviral regimens in southern Taiwan
- PMID: 30122963
- PMCID: PMC6082324
- DOI: 10.2147/IDR.S165811
HIV-1 genotypic drug resistance in patients with virological failure to single-tablet antiretroviral regimens in southern Taiwan
Abstract
Purpose: Sparse data are available on the prevalence of resistance among HIV-1-infected patients with virological failure to a single-tablet regimen (STR). This study aimed to evaluate the prevalence of HIV genotypic drug resistance in HIV-1-infected patients with virological failure to STRs in southern Taiwan.
Patients and methods: This retrospective study investigated drug resistance in patients with virological failure to STR from January 2016 to September 2017. Antiretroviral resistance mutations were defined using the 2017 International AIDS Society-USA HIV drug resistance algorithm, and drug resistance was compared using the HIVdb program of the Stanford University HIV Drug Resistance Database. Variables between resistance and non-resistance groups were compared.
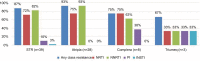
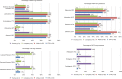
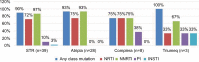
Results: Thirty-nine HIV-1-infected patients with treatment failure were tested for resistance, of whom 89% were infected by men who have sex with men. Subtype B HIV-1 strains were found in 90% of the patients. Eight patients were treatment naïve and initiated STRs, while 31 patients experienced treatment failure after switching to STRs. Eighty-seven percent of the patients harbored any of four classes of resistance (nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, protease inhibitors (PIs), and integrase strand transfer inhibitors). The prevalence rates of nucleoside reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, PI, and integrase strand transfer inhibitor resistance were 72%, 82%, 10%, and 3%, respectively. Patients with PI resistance were more likely to respond to treatment with a non-tenofovir disoproxil fumarate/emtricitabine/efavirenz-based STR (.=0.004) and a longer duration of antiretroviral therapy (101 months [72.0-123.3] vs 11 months [7-44], P=0.007). There were no associations between different STRs and transmission risk factors, HIV subtype, duration of antiretroviral therapy, and resistance to tenofovir disoproxil fumarate.
Conclusion: A high rate of antiretroviral drug resistance was found in the patients who failed STR treatment. The presence of PI resistance in these patients represented an inappropriate switch from a multiple tablet regimen to an STR. These findings should remind clinicians that detailed drug resistance history and close monitoring are mandatory after switching to an STR.
Keywords: HIV; antiretroviral therapy; drug resistance; single-tablet regimen; treatment failure.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
High Prevalence of Doravirine Resistance in HIV-1-Infected Patients with Virological Failure to an NNRTI-Based Single-Tablet Regimen.Infect Drug Resist. 2022 Jul 20;15:3857-3869. doi: 10.2147/IDR.S361012. eCollection 2022. Infect Drug Resist. 2022. PMID: 35899084 Free PMC article.
-
Durability of single tablet regimen for patients with HIV infection in Southern Taiwan: data from a real-world setting.BMC Infect Dis. 2022 Jan 4;22(1):2. doi: 10.1186/s12879-021-06919-6. BMC Infect Dis. 2022. PMID: 34983388 Free PMC article.
-
Efficacy and safety of a single-tablet regimen containing tenofovir disoproxil fumarate 300 mg, lamivudine 300 mg and efavirenz 400 mg as a switch strategy in virologically suppressed HIV-1-infected subjects on nonnucleoside reverse transcriptase inhibitor-containing first-line antiretroviral therapy in Pune, India.HIV Med. 2020 Oct;21(9):578-587. doi: 10.1111/hiv.12912. Epub 2020 Jul 20. HIV Med. 2020. PMID: 33021066 Free PMC article.
-
Efavirenz/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (Atripla®): a review of its use in the management of HIV infection.Drugs. 2010 Dec 3;70(17):2315-38. doi: 10.2165/11203800-000000000-00000. Drugs. 2010. PMID: 21080746 Review.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
Cited by
-
Nucleic acid testing and molecular characterization of HIV infections.Eur J Clin Microbiol Infect Dis. 2019 May;38(5):829-842. doi: 10.1007/s10096-019-03515-0. Epub 2019 Feb 23. Eur J Clin Microbiol Infect Dis. 2019. PMID: 30798399 Review.
References
-
- Sterne JA, Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378–384. - PubMed
-
- Jin Y, Liu Z, Wang X, et al. A systematic review of cohort studies of the quality of life in HIV/AIDS patients after antiretroviral therapy. Int J STD AIDS. 2014;25(11):771–777. - PubMed
-
- Kumarasamy N, Solomon S, Chaguturu SK, et al. The changing natural history of HIV disease: before and after the introduction of generic antiretroviral therapy in southern India. Clin Infect Dis. 2005;41(10):1525–1528. - PubMed
-
- Taniguchi T, Nurutdinova D, Grubb JR, et al. Transmitted drug-resistant HIV type 1 remains prevalent and impacts virologic outcomes despite genotype-guided antiretroviral therapy. AIDS Res Hum Retroviruses. 2012;28(3):259–264. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

