Review of Drug Repositioning Approaches and Resources
- PMID: 30123072
- PMCID: PMC6097480
- DOI: 10.7150/ijbs.24612
Review of Drug Repositioning Approaches and Resources
Abstract
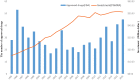
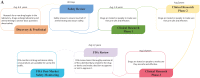
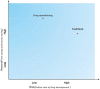
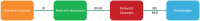
Drug discovery is a time-consuming, high-investment, and high-risk process in traditional drug development. Drug repositioning has become a popular strategy in recent years. Different from traditional drug development strategies, the strategy is efficient, economical and riskless. There are usually three kinds of approaches: computational approaches, biological experimental approaches, and mixed approaches, all of which are widely used in drug repositioning. In this paper, we reviewed computational approaches and highlighted their characteristics to provide references for researchers to develop more powerful approaches. At the same time, the important findings obtained using these approaches are listed. Furthermore, we summarized 76 important resources about drug repositioning. Finally, challenges and opportunities in drug repositioning are discussed from multiple perspectives, including technology, commercial models, patents and investment.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Sertkaya A, Birkenbach A, Berlind A, Eyraud J. Examination of clinical trial costs and barriers for drug development. US Department of health and human services, office of the assistant secretary for planning and evaluation report. 2014;1:1–92.
-
- Yeu Y, Yoon Y, Park S. Protein localization vector propagation: a method for improving the accuracy of drug repositioning. Mol Biosyst. 2015;11(7):2096–102. - PubMed
-
- Pharmaceutical Research and Manufacturers of America. http://www.phrma.org/sites/default/files/pdf/2015_phrma_profile.pdf.2015.
-
- Drug development process. https://www.fda.gov/Drugs/default.htm.
-
- Drug approval process. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/default.htm.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

