Hydrogen Peroxide and Abscisic Acid Mediate Salicylic Acid-Induced Freezing Tolerance in Wheat
- PMID: 30123235
- PMCID: PMC6085453
- DOI: 10.3389/fpls.2018.01137
Hydrogen Peroxide and Abscisic Acid Mediate Salicylic Acid-Induced Freezing Tolerance in Wheat
Abstract
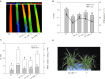
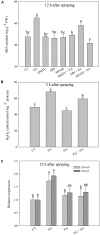
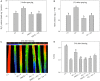
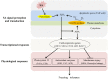
Salicylic acid (SA) can induce plant resistance to biotic and abiotic stresses through cross talk with other signaling molecules, whereas the interaction between hydrogen peroxide (H2O2) and abscisic acid (ABA) in response to SA signal is far from clear. Here, we focused on the roles and interactions of H2O2 and ABA in SA-induced freezing tolerance in wheat plants. Exogenous SA pretreatment significantly induced freezing tolerance of wheat via maintaining relatively higher dark-adapted maximum photosystem II quantum yield, electron transport rates, less cell membrane damage. Exogenous SA induced the accumulation of endogenous H2O2 and ABA. Endogenous H2O2 accumulation in the apoplast was triggered by both cell wall peroxidase and membrane-linked NADPH oxidase. The pharmacological study indicated that pretreatment with dimethylthiourea (H2O2 scavenger) completely abolished SA-induced freezing tolerance and ABA synthesis, while pretreatment with fluridone (ABA biosynthesis inhibitor) reduced H2O2 accumulation by inhibiting NADPH oxidase encoding genes expression and partially counteracted SA-induced freezing tolerance. These findings demonstrate that endogenous H2O2 and ABA signaling may form a positive feedback loop to mediate SA-induced freezing tolerance in wheat.
Keywords: endogenous signal; freezing tolerance; inhibitor; salicylic acid; wheat.
Figures



References
-
- Adam A. L., Bestwick C. S., Barna B., Mansfield J. W. (1995). Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv. phaseolicola. Planta 197 240–249. 10.1007/BF00202643 - DOI
-
- Agarwala S., Sairama R. K., Srivastavaa G. C., Tyagib A., Meena R. C. (2005). Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 169 559–570. 10.1016/j.plantsci.2005.05.004 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

