Length variations within the Merle retrotransposon of canine PMEL: correlating genotype with phenotype
- PMID: 30123327
- PMCID: PMC6091007
- DOI: 10.1186/s13100-018-0131-6
Length variations within the Merle retrotransposon of canine PMEL: correlating genotype with phenotype
Abstract
Background: The antisense insertion of a canine short interspersed element (SINEC_Cf) in the pigmentation gene PMEL (or SILV) causes a coat pattern phenotype in dogs termed merle. Merle is a semi-dominant trait characterized by patches of full pigmentation on a diluted background. The oligo(dT) tract of the Merle retrotransposon is long and uninterrupted and is prone to dramatic truncation. Phenotypically wild-type individuals carrying shorter oligo(dT) lengths of the Merle allele have been previously described and termed cryptic merles. Two additional coat patterns, dilute merle (uniform, steely-grey coat) and harlequin merle (white background with black patches), also appear in breeds segregating the Merle allele.
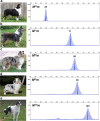
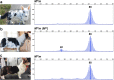
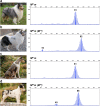
Results: Sequencing of all PMEL exons in a dilute and a harlequin merle reveals that variation exists solely within the oligo(dT) tract of the SINEC_Cf insertion. In fragment analyses from 259 dogs heterozygous for Merle, we observed a spectrum of oligo(dT) lengths spanning 25 to 105 base pairs (bp), with ranges that correspond to the four varieties of the merle phenotype: cryptic (25-55 bp), dilute (66-74 bp), standard (78-86 bp), and harlequin (81-105 bp). Somatic contractions of the oligo(dT) were observed in 43% of standard and 51% of harlequin merle dogs. A small proportion (4.6%) of the study cohort inherited de novo contractions or expansions of the Merle allele that resulted in dilute or harlequin coat patterns, respectively.
Conclusions: The phenotypic consequence of the Merle SINE insertion directly depends upon oligo(dT) length. In transcription, we propose that the use of an alternative splice site increases with oligo(dT) length, resulting in insufficient PMEL and a pigment dilution spectrum, from dark grey to complete hypopigmentation. We further propose that during replication, contractions and expansions increase in frequency with oligo(dT) length, causing coat variegation (somatic events in melanocytes) and the spontaneous appearance of varieties of the merle phenotype (germline events).
Keywords: Alternative splicing; Coat pattern; Dilution; Dog; Exonization; Mononucleotide repeat; Pigmentation; SILV; SINE; Slippage.
Conflict of interest statement
All samples were obtained with informed owner consent according to protocols approved by the Clemson University Institutional Review Board (IBC2008–17) and IACUC (2012–039). Not applicable. The authors declare that they have no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Sorsby A, Davey JB. Ocular associations of dappling (or merling) in the coat colour of dogs. J Genet. 1954;52:425. doi: 10.1007/BF02981535. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

