Risk of Cardiovascular Events Associated with Inhaled Corticosteroid Treatment in Patients with Chronic Obstructive Pulmonary Disease: A Meta-Analysis
- PMID: 30123392
- PMCID: PMC6079461
- DOI: 10.1155/2018/7097540
Risk of Cardiovascular Events Associated with Inhaled Corticosteroid Treatment in Patients with Chronic Obstructive Pulmonary Disease: A Meta-Analysis
Abstract
Background: The cardiovascular (CV) safety of inhaled corticosteroids (ICSs) in chronic obstructive pulmonary disease (COPD) is controversial because different studies have suggested that ICSs either increase or reduce the risk of CV events in COPD patients. In this meta-analysis, we assess the CV safety of ICS therapy in COPD.
Methods: A meta-analysis of randomized, double-blind, parallel-group, placebo-controlled trials of ICS treatment for COPD that include at least 4 weeks of follow-up was performed. A random-effects model was used to evaluate the effects of ICS treatment on CV events. CV events were documented in each trial, and the relative risk (RR) and 95% confidence intervals (CIs) for ICSs were estimated.
Results: Thirty-one trials were included in this meta-analysis. The risk of CV events was not different between ICS-treated and control groups (RR: 0.99; 95% CI: 0.93 to 1.06; P=0.801). In a subgroup analysis, there were no significant differences in CV events between an ICS combined with long-acting β2 agonist (LABA) (ICS + LABA) group and an LABA-only group (RR: 1.00; 95% CI: 0.90 to 1.10; P=0.930), as well as between a combination group (ICS + LABA) and a long-acting muscarinic antagonist (LAMA) combined with LABA (LAMA + LABA) group (RR: 0.78; 95% CI: 0.39 to 1.55; P=0.473). In addition, there was no difference in the risk of CV events between ICS treatment and control groups (RR: 0.99; 95% CI: 0.90 to 1.09; P=0.872).
Conclusions: These results demonstrate that ICSs do not increase the risk of CV events in COPD patients.
Figures
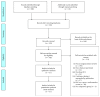


References
-
- Collaborators GBDCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Respiratory Medicine. 2017;5:691–706. - PMC - PubMed
-
- Dransfield M. T., Bourbeau J., Jones P. W., et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. The Lancet Respiratory Medicine. 2013;1(3):210–223. doi: 10.1016/s2213-2600(13)70040-7. - DOI - PubMed
-
- Stolz D., Hirsch H. H., Schilter D., et al. Intensified therapy with inhaled corticosteroids and LABA at the onset of URTI to prevent COPD exacerbations-a multicentre, randomised, double-blind, placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine. 2017;197(9):1136–1146. doi: 10.1164/rccm.201709-1807oc. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

