A randomized placebo-controlled phase II study of clarithromycin or placebo combined with VCD induction therapy prior to high-dose melphalan with stem cell support in patients with newly diagnosed multiple myeloma
- PMID: 30123673
- PMCID: PMC6090810
- DOI: 10.1186/s40164-018-0110-0
A randomized placebo-controlled phase II study of clarithromycin or placebo combined with VCD induction therapy prior to high-dose melphalan with stem cell support in patients with newly diagnosed multiple myeloma
Abstract
Background: The objective of this randomized placebo-controlled study was to investigate the efficacy and safety of clarithromycin in combination with bortezomib-cyclophosphamide-dexamethasone (VCD) in patients with newly diagnosed multiple myeloma eligible for high-dose therapy.
Methods: Patients were randomized to receive tablet clarithromycin 500 mg or matching placebo tablet twice daily during the first 3 cycles of VCD induction therapy. Primary endpoint was to compare the rate of very good partial response (VGPR) or better response after three cycles of VCD combined with clarithromycin or placebo.
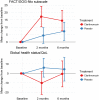
Results: The study was prematurely stopped for safety reasons after the inclusion of 58 patients (36% of the planned study population). The patients were randomly assigned to clarithromycin (n = 27) or placebo (n = 31). VGPR or better response after the VCD induction therapy was obtained in 12 patients (44.4%, 95% CI 25.5-64.7) and in 16 patients (51.6%, 33.1-69.8) (p = 0.59) in the clarithromycin group and the placebo group, respectively. Seven patients (25.9%) in the clarithromycin group developed severe gastrointestinal complications (≥ grade 3) comprising pain, neutropenic enterocolitis, paralytic ileus or peptic ulcer. These complications occurred in only one patient in the placebo group. Septicemia with Gram negative bacteria was observed in 5 patients in the clarithromycin group in contrast to one case of pneumococcal septicemia in the placebo group. Patient-reported QoL were negatively affected in the clarithromycin group compared to the placebo group.
Conclusion: The study was prematurely stopped due to serious adverse events, in particular serious gastrointestinal complications and septicemia. The response data do not suggest any effect of clarithromycin when added to the VCD regimen. The combination of clarithromycin and bortezomib containing regimens is toxic and do not seem to offer extra anti-myeloma efficacy.Trial registration EudraCT (no. 2014-002187-32, registered 7 October 2014, https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-002187-32/DK) and ClinicalTrials.gov (no NCT02573935, retrospectively registered 12 October 2015, https://www.clinicaltrials.gov/ct2/show/NCT02573935?term=Gregersen&cntry=DK&rank=9).
Keywords: Adverse drug event; Bortezomib; Clarithromycin; Double blind study; Induction chemotherapy; Multiple myeloma.
Figures
References
-
- Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111(3):1101–1109. doi: 10.1182/blood-2007-05-090258. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

