Isolation of circulating tumor cells in a preclinical model of osteosarcoma: Effect of chemotherapy
- PMID: 30123735
- PMCID: PMC6092555
- DOI: 10.1016/j.jbo.2018.07.002
Isolation of circulating tumor cells in a preclinical model of osteosarcoma: Effect of chemotherapy
Abstract
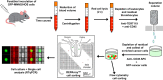
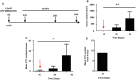
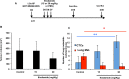
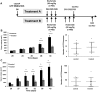
Osteosarcoma is a rare primary bone tumor, which mainly affects children and adolescents and has a poor prognosis, especially for patients with metastatic disease. A poor therapeutic response to the conventional chemotherapy is observed with the development of lung metastases, highlighting the need for improving the current regimens and the identification of early markers of the recurrent and metastatic disease. Circulating Tumour Cells (CTCs) play a key role in the metastatic process and could be powerful biomarkers of the progressive disease. The present study aimed to isolate CTCs by using a pre-clinical model of human osteosarcoma and to monitor their kinetic of release and their modulation by ifosfamide. CTCs were detectable into the bloodstream before any palpable primary tumors. Ifosfamide increased CTCs count and in contrast decreased the number of lung tumor nodules. On established tumors, ifosfamide slowed down the tumour growth and did not modulate CTC count that could be explained by a release of cancer cells from the primary tumour with reduced properties for inducing lung metastases. This report highlights the biological interest of CTCs in osteosarcoma.
Keywords: Biomarker; Circulating tumor cells; Metastatic process; Osteosarcoma; Recurrent disease.
Figures
References
-
- Mohseny A.B., Szuhai K., Romeo S., Buddingh A.P., Briaire-de Bruijn I., de Jong D., van Pel M., Cleton-Jansen A.M., Hogendoorn P.C. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J. Pathol. 2009;219:294–305. - PubMed
-
- Mutsaers A.J., Walkley C.R. Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone. 2014;62:56–63. - PubMed
-
- Heymann M.F., Brown H.K., Heymann D. Drugs in early clinical development for the treatment of osteosarcoma. Expert Opin. Investig. Drugs. 2016;25:1265–1280. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

