Dravet Syndrome: A Sodium Channel Interneuronopathy
- PMID: 30123852
- PMCID: PMC6091224
- DOI: 10.1016/j.cophys.2017.12.007
Dravet Syndrome: A Sodium Channel Interneuronopathy
Abstract
Dravet Syndrome is a devastating childhood epilepsy disorder with high incidence of premature death plus comorbidities of ataxia, circadian rhythm disorder, impaired sleep quality, autistic-like social-interaction deficits and severe cognitive impairment. It is primarily caused by heterozygous loss-of-function mutations in the SCN1A gene that encodes brain voltage-gated sodium channel type-1, termed NaV1.1. Here I review experiments on mouse genetic models that implicate specific loss of sodium currents and action potential firing in GABAergic inhibitory interneurons as the fundamental cause of Dravet Syndrome. The resulting imbalance of excitatory to inhibitory neurotransmission in neural circuits causes both epilepsy and co-morbidities. Promising therapeutic approaches involving atypical sodium channel blockers, novel drug combinations, and cannabidiol give hope for improved outcomes for Dravet Syndrome patients.
Keywords: Epilepsy; autism; cognitive deficit; interneurons; sodium channels.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

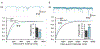
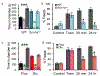


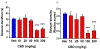
References
-
- Hille B: Ionic Channels of Excitable Membranes edn 3rd Ed Sunderland, MA: Sinauer Associates Inc; 2001.
-
- Catterall WA: From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 2000, 26:13–25. - PubMed
-
- Hille B: Ionic Channels of Excitable Membranes, 3rd Ed Sunderland, MA: Sinauer Associates Inc; 2001.
-
- Vilin YY, Ruben PC: Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem Biophys 2001, 35:171–190. - PubMed
-
- Dravet C: The core Dravet syndrome phenotype. Epilepsia 2011, 52 Suppl 2:3–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
