Force-transmitting structures in the digital pads of the tree frog Hyla cinerea: a functional interpretation
- PMID: 30123974
- PMCID: PMC6131963
- DOI: 10.1111/joa.12860
Force-transmitting structures in the digital pads of the tree frog Hyla cinerea: a functional interpretation
Abstract
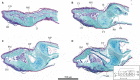
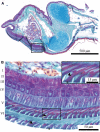
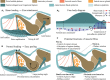
The morphology of the digital pads of tree frogs is adapted towards attachment, allowing these animals to attach to various substrates and to explore their arboreal habitat. Previous descriptions and functional interpretations of the pad morphology mostly focussed on the surface of the ventral epidermis, and little is known about the internal pad morphology and its functional relevance in attachment. In this study, we combine histology and synchrotron micro-computer-tomography to obtain a comprehensive 3-D morphological characterisation of the digital pads (in particular of the internal structures involved in the transmission of attachment forces from the ventral pad surface towards the phalanges) of the tree frog Hyla cinerea. A collagenous septum runs from the distal tip of the distal phalanx to the ventral cutis and compartmentalises the subcutaneous pad volume into a distal lymph space and a proximal space, which contains mucus glands opening via long ducts to the ventral pad surface. A collagen layer connects the ventral basement membrane via interphalangeal ligaments with the middle phalanx. The collagen fibres forming this layer curve around the transverse pad-axis and form laterally separated ridges below the gland space. The topological optimisation of a shear-loaded pad model using finite element analysis (FEA) shows that the curved collagen fibres are oriented along the trajectories of the maximum principal stresses, and the optimisation also results in ridge-formation, suggesting that the collagen layer is adapted towards a high stiffness during shear loading. We also show that the collagen layer is strong, with an estimated tensile strength of 2.0-6.5 N. Together with longitudinally skewed tonofibrils in the superficial epidermis, these features support our hypothesis that the digital pads of tree frogs are primarily adapted towards the generation and transmission of friction rather than adhesion forces. Moreover, we generate (based on a simplified FEA model and predictions from analytical models) the hypothesis that dorsodistal pulling on the collagen septum facilitates proximal peeling of the pad and that the septum is an adaptation towards detachment rather than attachment. Lastly, by using immunohistochemistry, we (re-)discovered bundles of smooth muscle fibres in the digital pads of tree frogs. We hypothesise that these fibres allow the control of (i) contact stresses at the pad-substrate interface and peeling, (ii) mucus secretion, (iii) shock-absorbing properties of the pad, and (iv) the macroscopic contact geometry of the ventral pad surface. Further work is needed to conclude on the role of the muscular structures in tree frog attachment. Overall, our study contributes to the functional understanding of tree frog attachment, hence offering novel perspectives on the ecology, phylogeny and evolution of anurans, as well as the design of tree-frog-inspired adhesives for technological applications.
Keywords: attachment organ; bioadhesion; collagen; connective tissue; fibre-matrix-composite; material stiffness; shear load; smooth muscle.
© 2018 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures








References
-
- Afferante L, Heepe L, Casdorff K, et al. (2016) A theoretical characterization of curvature controlled adhesive properties of bio‐inspired membranes. Biomimetics 1, 3.
-
- Autumn K, Dittmore A, Santos D, et al. (2006) Frictional adhesion: a new angle on gecko attachment. J Exp Biol 209, 3569–3579. - PubMed
-
- Barnes WJP (2012) Adhesion in wet environments: frogs In: Encyclopedia of Nanotechnology. (ed. Bhushan B.), pp. 70–83. New York, NY: Springer.
-
- Barnes WJP, Baum M, Peisker H, et al. (2013) Comparative cryo‐SEM and AFM studies of hylid and rhacophorid tree frog toe pads. J Morphol 274, 1384–1396. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

