UV Resistance of bacteria from the Kenyan Marine cyanobacterium Moorea producens
- PMID: 30123980
- PMCID: PMC6460272
- DOI: 10.1002/mbo3.697
UV Resistance of bacteria from the Kenyan Marine cyanobacterium Moorea producens
Abstract
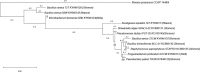
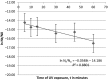
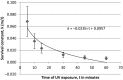
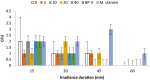
UV resistance of bacteria isolated from the marine cyanobacterium Moorea producens has not been observed previously, findings which highlight how unsafe germicidal UV irradiation for sterilization of air, food, and water could be. Further, UV resistance of Bacillus licheniformis is being observed for the first time. This study focused on bacteria isolated from the marine cyanobacterium M. producens collected off the Kenyan coast at Shimoni, Wasini, Kilifi, and Mida. UV irradiance of isolates (302 nm, 70 W/m2 , 0-1 hr) established B. licheniformis as the most UV resistant strain, with the following order of taxon resistance: Bacilli> γ proteobacteria > Actinobacteria. UV resistance was independent of pigmentation. The maximum likelihood phylogenetic distance determined for both B. licheniformis and Bacillus aerius relative to M. producens CCAP 1446/4 was 2.0. Survival of B. licheniformis upon UV irradiance followed first-order kinetics (k = 0.035/min, R2 = 0.88). Addition of aqueous extracts (2, 10, 20 and 40 mg/ml) of this B. licheniformis strain on the less resistant Marinobacterium stanieri was not significant, however, the commercial sunscreen benzophenone-3 (BP-3) positive control and the time of irradiance were significant. Detection of bacteria on M. producens filaments stained with acridine orange confirmed its nonaxenic nature. Although the chemistry of UV resistance in cyanobacteria has been studied in depth revealing for example the role of mycosporine like amino acids (MAAs) in UV resistance less is known about how bacteria resist UV irradiation. This is of interest since cyanobacteria live in association with bacteria.
Keywords: Bacillus licheniformis; Moorea producens; UV resistance; bacteria; benzophenone-3; first-order kinetics.
© 2018 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Bandaranayake, W. M. , Bemis, J. E. , & Bourne, D. J. (1996). Ultraviolet absorbing pigments from the marine sponge Dysidea herbacea: isolation and structure of a new mycosporine. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 115(3), 281–286.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

