Building a functional connectome of the Drosophila central complex
- PMID: 30124430
- PMCID: PMC6150698
- DOI: 10.7554/eLife.37017
Building a functional connectome of the Drosophila central complex
Abstract
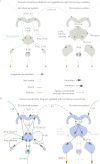
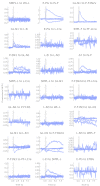
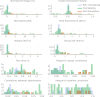
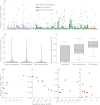
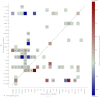
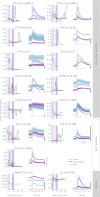
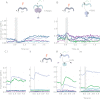
The central complex is a highly conserved insect brain region composed of morphologically stereotyped neurons that arborize in distinctively shaped substructures. The region is implicated in a wide range of behaviors and several modeling studies have explored its circuit computations. Most studies have relied on assumptions about connectivity between neurons based on their overlap in light microscopy images. Here, we present an extensive functional connectome of Drosophila melanogaster's central complex at cell-type resolution. Using simultaneous optogenetic stimulation, calcium imaging and pharmacology, we tested the connectivity between 70 presynaptic-to-postsynaptic cell-type pairs. We identified numerous inputs to the central complex, but only a small number of output channels. Additionally, the connectivity of this highly recurrent circuit appears to be sparser than anticipated from light microscopy images. Finally, the connectivity matrix highlights the potentially critical role of a class of bottleneck interneurons. All data are provided for interactive exploration on a <ext-link ext-link-type="uri" xlink:href="https://romainfr.github.io/CX-Functional-Website/">website</ext-link>.
Keywords: D. melanogaster; central complex; functional connectivity; neuroscience; open data; optogenetics; ring attractor; two-photon calcium imaging.
© 2018, Franconville et al.
Conflict of interest statement
RF, CB, VJ No competing interests declared
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

