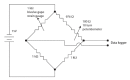
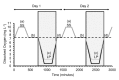
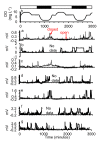
A Strain Gauge Monitor (SGM) for Continuous Valve Gape Measurements in Bivalve Molluscs in Response to Laboratory Induced Diel-cycling Hypoxia and pH
- PMID: 30124658
- PMCID: PMC6126594
- DOI: 10.3791/57404
A Strain Gauge Monitor (SGM) for Continuous Valve Gape Measurements in Bivalve Molluscs in Response to Laboratory Induced Diel-cycling Hypoxia and pH
Abstract
An inexpensive, laboratory-based, strain gauge valve gape monitor (SGM) was developed to monitor the valve gape behavior of bivalve molluscs in response to diel-cycling hypoxia. A Wheatstone bridge was connected to strain gauges that were attached to the shells of oysters (Crassostrea virginica). The recorded signals allowed for the opening and closing of the bivalves to be recorded continuously over two-day periods of experimentally-induced diel-cycling hypoxia and diel-cycling changes in pH. Here, we describe a protocol for developing an inexpensive strain gauge monitor and describe, in an example laboratory experiment, how we used it to measure the valve gape behavior of Eastern oysters (C. virginica), in response to diel-cycling hypoxia and cyclical changes in pH. Valve gape was measured on oysters subjected to cyclical severe hypoxic (0.6 mg/L) dissolved oxygen conditions with and without cyclical changes in pH, cyclical mild hypoxic (1.7 mg/L) conditions and normoxic (7.3 mg/L) conditions. We demonstrate that when oysters encounter repeated diel cycles, they rapidly close their shells in response to severe hypoxia and close with a time lag to mild hypoxia. When normoxia is restored, they rapidly open again. Oysters did not respond to cyclical pH conditions superimposed on diel cycling severe hypoxia. At reduced oxygen conditions, more than one third of the oysters closed simultaneously. We demonstrate that oysters respond to diel-cycling hypoxia, which must be considered when assessing the behavior of bivalves to dissolved oxygen. The valve SGM can be used to assess responses of bivalve molluscs to changes in dissolved oxygen or contaminants. Sealing techniques to better seal the valve gape strain gauges from sea water need further improvement to increase the longevity of the sensors.
References
-
- Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. - PubMed
-
- Levin LA, Breitburg DL. Linking coasts and seas to address ocean deoxygenation. Nature Climate Change. 2015;5:401–403.
-
- Diaz RJ, Rosenberg R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: An annual Review. 1995;33:245–303.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources