ADAM8 localizes to extravillous trophoblasts within the maternal-fetal interface and potentiates trophoblast cell line migration through a β1 integrin-mediated mechanism
- PMID: 30124911
- PMCID: PMC6154767
- DOI: 10.1093/molehr/gay034
ADAM8 localizes to extravillous trophoblasts within the maternal-fetal interface and potentiates trophoblast cell line migration through a β1 integrin-mediated mechanism
Abstract
Study question: Does A Disintegrin And Metalloproteinase 8 (ADAM8) control extravillous trophoblast (EVT) differentiation and migration in early human placental development?
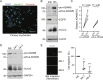
Summary answer: ADAM8 mRNA preferentially localizes to invasive HLA-G-positive trophoblasts, associates with the acquirement of an EVT phenotype and promotes trophoblast migration through a mechanism requiring β1-integrin.
What is known already: Placental establishment in the first trimester of pregnancy requires the differentiation of progenitor trophoblasts into invasive EVTs that produce a diverse repertoire of proteases that facilitate matrix remodeling and activation of signaling pathways important in controlling cell migration. While multiple ADAM proteases, including ADAM8, are highly expressed by invasive trophoblasts, the role of ADAM8 in controlling EVT-related processes is unknown.
Study design, size, duration: First trimester placental villi and decidua (6-12 weeks' gestation), primary trophoblasts and trophoblastic cell lines (JEG3, JAR, Bewo, HTR8/SVNeo) were used to examine ADAM8 expression, localization and function. All experiments were performed on at least three independent occasions (n = 3).
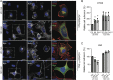
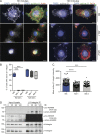
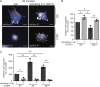
Participants/materials, setting, methods: Placental villi and primary trophoblasts derived from IRB approved first trimester placental (n = 24) and decidual (n = 4) were used to examine ADAM8 localization and expression by in situ RNAScope hybridization, flow cytometry, quantitative PCR and immunoblot analyses. Primary trophoblasts were differentiated into EVT-like cells by plating on fibronectin and were assessed by immunofluorescence microscopy and immunoblot analysis of keratin-7, vimentin, epidermal growth factor receptor (EGFR), HLA-G and ADAM8. ADAM8 function was examined in primary EVTs and trophoblastic cell lines utilizing siRNA-directed silencing and over-expression strategies. Trophoblast migration was assessed using Transwell chambers, cell-matrix binding was tested using fibronectin-adhesion assays, and ADAM8-β1-integrin interactions were determined by immunofluorescence microscopy, co-immunoprecipitation experiments and function-promoting/inhibiting antibodies.
Main results and the role of chance: Within first trimester placental tissues, ADAM8 preferentially localized to HLA-G+ trophoblasts residing within anchoring columns and decidua. Functional experiments in primary trophoblasts and trophoblastic cell lines show that ADAM8 promotes trophoblast migration through a mechanism independent of intrinsic protease activity. We show that ADAM8 localizes to peri-nuclear and cell-membrane actin-rich structures during cell-matrix attachment and promotes trophoblast binding to fibronectin matrix. Moreover, ADAM8 potentiates β1-integrin activation and promotes cell migration through a mechanism dependent on β1-integrin function.
Limitations, reasons for caution: The primary limitation of this study was the use of in vitro experiments in examining ADAM8 function, as well as the implementation of immortalized trophoblastic cell lines. Histological localization of ADAM8 within placental and decidual tissue sections was limited to mRNA level analysis. Further, patient information corresponding to tissues obtained by elective terminations was not available.
Wider implications of the findings: The novel non-proteolytic pro-migratory role for ADAM8 in controlling trophoblast migration revealed by this study sheds insight into the importance of ADAM8 in EVT biology and placental development.
Study funding/competing interest(s): This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC-Discovery Grant) and the Canadian Institutes of Health Research (CIHR-Open Operating Grant). There are no conflicts or competing interests.
Trial registration number: NA.
Figures


Similar articles
-
Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions.Hum Reprod. 2017 Jun 1;32(6):1208-1217. doi: 10.1093/humrep/dex058. Hum Reprod. 2017. PMID: 28369440
-
Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor.Hum Reprod. 2017 Jun 1;32(6):1218-1229. doi: 10.1093/humrep/dex069. Hum Reprod. 2017. PMID: 28402449 Free PMC article.
-
ADAM28 localizes to HLA-G+ trophoblasts and promotes column cell outgrowth.Placenta. 2017 Jul;55:71-80. doi: 10.1016/j.placenta.2017.05.009. Epub 2017 May 17. Placenta. 2017. PMID: 28623976
-
Investigation of human trophoblast invasion in vitro.Hum Reprod Update. 2020 Jun 18;26(4):501-513. doi: 10.1093/humupd/dmaa017. Hum Reprod Update. 2020. PMID: 32441309 Free PMC article. Review.
-
Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies.Hum Reprod Update. 2018 Jul 1;24(4):484-496. doi: 10.1093/humupd/dmy008. Hum Reprod Update. 2018. PMID: 29608700 Free PMC article. Review.
Cited by
-
Palmitic Acid Impedes Extravillous Trophoblast Activity by Increasing MRP1 Expression and Function.Biomolecules. 2022 Aug 22;12(8):1162. doi: 10.3390/biom12081162. Biomolecules. 2022. Retraction in: Biomolecules. 2024 Apr 08;14(4):453. doi: 10.3390/biom14040453. PMID: 36009056 Free PMC article. Retracted.
-
Activin A increases human trophoblast invasion by upregulating integrin β1 through ALK4.FASEB J. 2021 Feb;35(2):e21220. doi: 10.1096/fj.202001604R. Epub 2020 Nov 23. FASEB J. 2021. PMID: 33230889 Free PMC article.
-
Mitochondrial Dynamics in Placenta-Derived Mesenchymal Stem Cells Regulate the Invasion Activity of Trophoblast.Int J Mol Sci. 2020 Nov 14;21(22):8599. doi: 10.3390/ijms21228599. Int J Mol Sci. 2020. PMID: 33202697 Free PMC article.
-
ADAM and ADAMTS disintegrin and metalloproteinases as major factors and molecular targets in vascular malfunction and disease.Adv Pharmacol. 2022;94:255-363. doi: 10.1016/bs.apha.2021.11.002. Epub 2022 Jan 24. Adv Pharmacol. 2022. PMID: 35659374 Free PMC article.
-
ADAM8 silencing suppresses the migration and invasion of fibroblast-like synoviocytes via FSCN1/MAPK cascade in osteoarthritis.Arthritis Res Ther. 2024 Jan 13;26(1):20. doi: 10.1186/s13075-023-03238-w. Arthritis Res Ther. 2024. PMID: 38218854 Free PMC article.
References
-
- Aghababaei M, Beristain AG. The Elsevier Trophoblast Research Award Lecture: importance of metzincin proteases in trophoblast biology and placental development: a focus on ADAM12. Placenta 2015;36:S11–S19. - PubMed
-
- Aghababaei M, Perdu S, Irvine K, Beristain AG. A disintegrin and metalloproteinase 12 (ADAM12) localizes to invasive trophoblast, promotes cell invasion and directs column outgrowth in early placental development. Mol Hum Reprod 2014;20:235–249. - PubMed
-
- Bax DV, Messent AJ, Tart J, van Hoang M, Kott J, Maciewicz RA, Humphries MJ. Integrin alpha5beta1 and ADAM-17 interact in vitro and co-localize in migrating HeLa cells. J Biol Chem 2004;279:22377–22386. American Society for Biochemistry and Molecular Biology. - PubMed
-
- Beristain AG, Molyneux SD, Joshi PA, Pomroy NC, Di Grappa MA, Chang MC, Kirschner LS, Privé GG, Pujana MA, Khokha R. PKA signaling drives mammary tumorigenesis through Src. Oncogene 2015;34:1160–1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

