Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial
- PMID: 30125052
- PMCID: PMC6489732
- DOI: 10.1002/clc.23055
Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial
Abstract
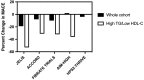
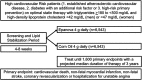
It is uncertain whether omega-3 fatty acids are beneficial in statin-treated patients. Epanova is a mix of omega-3 free fatty acids, not requiring co-ingestion with food, which can lower triglycerides by up to 31%. STRENGTH will examine whether Epanova 4 g daily reduces the rate of cardiovascular events in statin-treated patients with hypertriglyceridemia and low levels of HDL-C at high risk for developing cardiovascular events. STRENGTH is a randomized, double-blind, placebo-controlled trial. Patients had a triglyceride level ≥ 180 to <500 mg/dL and HDL-C < 42 mg/dL (men) or < 47 mg/dL (women) in the presence of either (1) established atherosclerotic cardiovascular disease, (2) diabetes with one additional risk factor, or (3) were other high-risk primary prevention patients, based on age and risk factor assessment. Patients should be treated with a statin, for >4 weeks, and have LDL-C < 100 mg/dL, but were also eligible if LDL-C was ≥100 mg/dL while on maximum tolerated statin therapy. The study will extend from October 30, 2014 to October 30, 2019. 13 086 patients were randomized to Epanova 4 g or placebo daily in addition to standard medical therapy. The primary efficacy outcome is time to first event of cardiovascular death, myocardial infarction, stroke, coronary revascularization or hospitalization for unstable angina. The trial will continue until 1600 patients reach the primary endpoint, with a median duration of therapy of 3 years. STRENGTH will determine whether Epanova 4 g daily will reduce cardiovascular events in statin-treated high-risk patients with hypertriglyceridemia and low HDL-C levels.
Keywords: cardiovascular risk; clinical trials; non-HDL cholesterol; omega-3 fatty acids; triglycerides.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Stephen J. Nicholls has received research support: AstraZeneca, Amgen, Anthera, Eli Lilly, Esperion, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi‐Regeneron, and LipoScience and is a consultant for AstraZeneca, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi‐Regeneron, CSL Behring, Esperion, Boehringer Ingelheim. A. Michael Lincoff reports receiving grant support, through a research contract with his institution, from Eli Lilly, AstraZeneca, Roche, CSL Behring, Esperion, and AbbVie. Christie M. Ballantyne received grant/research support (all significant [>$10 000] and paid to institution, not individual) from: Abbott Diagnostic, Amarin, Amgen Inc, Esperion, Ionis, Novartis, Pfizer, Regeneron, Roche Diagnostic, Sanofi‐Synthelabo, NIH, AHA, ADA; and served as a consultant (significant [>$10 000] denoted by *) for: Abbott Diagnostics, Amarin, Amgen Inc, AstraZeneca*, Boehringer Ingelheim*, Eli Lilly, Esperion, Ionis, Matinas BioPharma Inc, Merck*, Novartis, Pfizer*, Regeneron, Roche Diagnostic, Sanofi‐Synthelabo*. Philip J. Barter reports Research Grants received from Merck, Pfizer, honoraria received from Amgen, AstraZeneca, Merck, Pfizer, Sanofi‐Regeneron and is a member of Advisory Boards: Amgen, Merck, Pfizer, Sanofi‐Regeneron. Michael H. Davidson has received consultant and advisory board fees and honoraria and has participated in speaker's bureaus for AstraZeneca, Sanofi, Regeneron, and Amgen. He reports holding equity in Omthera Pharmaceuticals. John J. P. Kastelein received personal consulting fees from: Sanofi, Affiris, Akarna Therapeutics, Amgen Inc, CSL Behring, Regeneron, Staten Biotech, Madrigal, The Medicines Company, Kowa, Lilly, Esperion, Gemphire, Ionis Pharmaceuticals, Akcea Pharmaceuticals. Wolfgang Koenig reports modest consultation fees for advisory board meetings from Novartis, Pfizer, Kowa, Amgen, and AstraZeneca and modest personal fees for lectures from Novartis, Sanofi, and Amgen. Darren K. McGuire reports Clinical trial leadership: AstraZeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline, Esperion; Consultancy: AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk and Metavant. Dariush Mozaffarian reports personal fees from Acasti Pharma, AstraZeneca, GOED, DSM, Nutrition Impact, Pollock Communications, Bunge, and Indigo Agriculture; royalties from UpToDate for online medical chapters; scientific advisory board, Omada Health and Elysium Health; and research funding from the National Institutes of Health and the Gates Foundation; all outside the present work. Terje R. Pedersen reports grants and personal fees from Merck, Pfizer, and Amgen. Paul M. Ridke has received research grant support from Kowa, Novartis, and Pfizer and served as a consultant to AstraZeneca during the course of this trial. Kausik Ray reports grants and/or personal fees from Pfizer, MSD, AstraZeneca, Sanofi, Aegerion, Regeneron, Abbvie, Kowa, Cerenis, Medicines Company, Lilly, Esperion, Amgen, Cipla, Algorithm, Takeda, Boehringer Ingelheim, and Novo Nordisk within the last 12 months outside of the submitted work. Björn W. Karlson and Torbjörn Lundström are employees of AstraZeneca Pharmaceuticals. Steven E. Nissen reports that the Cleveland Clinic Center for Clinical Research has received funding to perform clinical trials from Abbvie, AstraZeneca, Amgen Inc, Cerenis, Eli Lilly, Esperion, Pfizer, The Medicines Company, Takeda, and Orexigen. Dr. Nissen is involved in these clinical trials, but receives no personal remuneration for his participation. Dr. Nissen consults for many pharmaceutical companies, but requires them to donate all honoraria or consulting fees directly to charity so that he receives neither income nor a tax deduction. Dianna Bash and Kathy Wolski have no industry relationships to disclose.
Figures
References
-
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225‐1228. - PubMed
-
- Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047‐2056. - PubMed
-
- Puri R, Nissen SE, Shao M, et al. Non‐HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol. 2016;36:2220‐2228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

