Lapatinib, a Dual Inhibitor of Epidermal Growth Factor Receptor (EGFR) and HER-2, Enhances Radiosensitivity in Mouse Bladder Tumor Line-2 (MBT-2) Cells In Vitro and In Vivo
- PMID: 30125265
- PMCID: PMC6113922
- DOI: 10.12659/MSM.909865
Lapatinib, a Dual Inhibitor of Epidermal Growth Factor Receptor (EGFR) and HER-2, Enhances Radiosensitivity in Mouse Bladder Tumor Line-2 (MBT-2) Cells In Vitro and In Vivo
Abstract
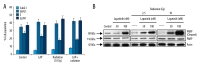
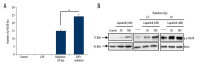
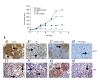
BACKGROUND The aim of this study was to evaluate the effect of lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR) and HER-2, on the radiosensitivity of murine bladder tumor line-2 (MBT-2) cells in vitro and in vivo. MATERIAL AND METHODS MBT-2 cells were pretreated with lapatinib at doses ranging from 200-1,000 nM for 30 min followed by radiation at doses ranging from 2.5-10 Gy for 30 min. A clonogenic assay (colony formation assay) assessed cell survival. Western blot measured phosphorylated epidermal growth factor receptor (p-EGFR), phosphorylated AKT (p-AKT), and phosphorylated HER-2 (p-HER2) and the apoptosis marker, PARP. The C3H/HeN mouse tumor xenograft model underwent subcutaneous injection of MBT-2 cells; mice were divided into four groups, treated with lapatinib (200 mg/kg), radiation (15 Gy), a combination of both, and with vehicle (control). RESULTS Lapatinib pretreatment, combined with radiation, decreased MBT-2 cell survival, and suppressed radiation-activated levels of p-EGFR and p-HER-2. MBT-2 cells treated with a 10 Gy dose of radiation and 1000 nM of lapatinib showed combination index (CI) values of <1 indicating synergy. Increased expression of γ-H2AX, indicated increased apoptosis. In mice with tumor xenografts, a daily dose of lapatinib (200 mg/kg/day) for seven days combined with radiation on the fourth day suppressed tumor growth to a greater degree than radiation alone. CONCLUSIONS Lapatinib treatment enhanced the radiation sensitivity in an in vitro and in vivo murine bladder cancer model by decreasing radiation-mediated EGFR and HER-2 activation, and by causing DNA damage leading to cell apoptosis.
Figures



Similar articles
-
Targeting epidermal growth factor receptor/human epidermal growth factor receptor 2 signalling pathway by a dual receptor tyrosine kinase inhibitor afatinib for radiosensitisation in murine bladder carcinoma.Eur J Cancer. 2013 Apr;49(6):1458-66. doi: 10.1016/j.ejca.2012.10.020. Epub 2012 Nov 12. Eur J Cancer. 2013. PMID: 23153706
-
Synergistic Blockade of EGFR and HER2 by New-Generation EGFR Tyrosine Kinase Inhibitor Enhances Radiation Effect in Bladder Cancer Cells.Mol Cancer Ther. 2015 Mar;14(3):810-20. doi: 10.1158/1535-7163.MCT-13-0951. Epub 2015 Jan 14. Mol Cancer Ther. 2015. PMID: 25589492
-
Antitumor and antiangiogenic effect of the dual EGFR and HER-2 tyrosine kinase inhibitor lapatinib in a lung cancer model.BMC Cancer. 2010 May 11;10:188. doi: 10.1186/1471-2407-10-188. BMC Cancer. 2010. PMID: 20459769 Free PMC article.
-
Dual/pan-HER tyrosine kinase inhibitors: focus in breast cancer.Adv Exp Med Biol. 2006;587:329-40. doi: 10.1007/978-1-4020-5133-3_25. Adv Exp Med Biol. 2006. PMID: 17163175 Review. No abstract available.
-
Epidermal growth factor receptor and its inhibition in radiotherapy: in vivo findings.Int J Radiat Biol. 2003 Jul;79(7):539-45. doi: 10.1080/0955300031000114747. Int J Radiat Biol. 2003. PMID: 14530163 Review.
Cited by
-
ASR488, a novel small molecule, activates an mRNA binding protein, CPEB1, and inhibits the growth of bladder cancer.Oncol Lett. 2020 Jul;20(1):850-860. doi: 10.3892/ol.2020.11593. Epub 2020 May 7. Oncol Lett. 2020. PMID: 32566012 Free PMC article.
-
Tocopherol-human serum albumin nanoparticles enhance lapatinib delivery and overcome doxorubicin resistance in breast cancer.Nanomedicine (Lond). 2024 Jul 2;19(16):1431-1448. doi: 10.1080/17435889.2024.2359357. Epub 2024 Jul 2. Nanomedicine (Lond). 2024. PMID: 38953854 Free PMC article.
-
Comparative effectiveness and tolerability of targeted agents combined with chemotherapy in patients with HER2-positive gastroesophageal cancer: A network meta-analysis.Saudi J Gastroenterol. 2022 May-Jun;28(3):175-185. doi: 10.4103/sjg.sjg_367_21. Saudi J Gastroenterol. 2022. PMID: 34747874 Free PMC article.
-
Review of Experimental Studies to Improve Radiotherapy Response in Bladder Cancer: Comments and Perspectives.Cancers (Basel). 2020 Dec 30;13(1):87. doi: 10.3390/cancers13010087. Cancers (Basel). 2020. PMID: 33396795 Free PMC article. Review.
-
Improving the Efficacy of Tumor Radiosensitization Through Combined Molecular Targeting.Front Oncol. 2020 Aug 4;10:1260. doi: 10.3389/fonc.2020.01260. eCollection 2020. Front Oncol. 2020. PMID: 32903756 Free PMC article. Review.
References
-
- Boyle P, Levin B, editors. International Agency for Research on Cancer. World Cancer Report. 2008. Available at: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscleinvasive and metastatic bladder cancer: Update of the EAU guidelines. Eur Urol. 2011;59(6):1009–18. - PubMed
-
- Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J Clin Oncol. 2002;20(14):3061–71. - PubMed
-
- Coppin CM, Gospodarowicz MK, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14(11):2901–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

