A 'Comprehensive Visual Rating Scale' for predicting progression to dementia in patients with mild cognitive impairment
- PMID: 30125290
- PMCID: PMC6101367
- DOI: 10.1371/journal.pone.0201852
A 'Comprehensive Visual Rating Scale' for predicting progression to dementia in patients with mild cognitive impairment
Abstract
Background: Numerous efforts have been made to identify biomarkers for predicting the progression of dementia in patients with mild cognitive impairment (MCI), and recently, a comprehensive visual rating scale (CVRS) based on magnetic resonance imaging (MRI) has been validated to assess structural changes in the brain of elderly patients. Based on this, the present study investigated the use of CVRS for predicting dementia and elucidated its association with cognitive change in patients with MCI over a three-year follow-up.
Methods: We included 340 patients with MCI with more than one follow-up visit. Data were obtained from the Alzheimer's disease Neuroimaging Initiative study. We assessed all the patients using CVRS and determined their progression to dementia during a follow-up period of over 3 years. The cox proportional hazards model was used to analyze hazard ratios (HRs) of CVRS for disease progression. Further, multiple cognitive measures of the patients over time were fitted using the random effect model to assess the effect of initial CVRS score on subsequent cognitive changes.
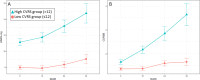
Results: Of 340 patients, 69 (20.2%) progressed to dementia and the median baseline score (interquartile range) of CVRS significantly differed between stable MCI and progressive MCI (9 (5-13) vs 13 (8-17), p<0.001). The initial CVRS score was independently associated with an increased risk of progression to dementia (HR 1.123, 95% confidence interval [CI] 1.059-1.192). From 12 to 24 months, the effect of the interaction between CVRS and interval of follow-up visit on cognitive performance achieved significance (p<0.001).
Conclusions: Baseline CVRS predicted the progression to dementia in patients with MCI, and was independently associated with longitudinal cognitive decline.
Conflict of interest statement
Funding for this work was derived in part from the following commercial sources: Araclon Biotech; BioClinica, Inc.;Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. Funding from these sources does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7: 270–279. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7: 280–292. 10.1016/j.jalz.2011.03.003 - DOI - PMC - PubMed
-
- Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7: 257–262. 10.1016/j.jalz.2011.03.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

